 CAC Antibody Collection
CAC Antibody Collection
The antibodies on this page are part of Cosmo Bio's exclusive CAC Collection. For many many thousands of other antibodies from many different makers, use our Search the Store function and our Explore Products drop down menu.
Extracellular matrix
Glycosaminoglycans (GAGs) / Proteoglycans / Matrix and basement membrane / Cell adhesion and hemidesmosome-related / Bone and cartilage-related / Wound repair-related
| Extracellular matrix: Glycosaminoglycans (GAGs) | |||
| Product name (click for order info) | Cat No (click for datasheet) |
Host | Species specificity |
| Anti Keratan Sulfate (KS/Keratosulfate) mAb (Clone 373E1) | CAC-PRPG-KS-M01 | RT | All Animals |
| Anti 4-Sulfated Unsaturated Disaccharide Neoepitopes (C-4-S "stubs") of Chondroitin Sulfate or Dermatan Sulfate mAb (Clone 2B6) | CAC-PRPG-BC-M02 | MS | All Animals |
| Anti Unsulfated Unsaturated Disaccharide Neoepitopes (C-0-S "stubs") of Chondroitin Sulfate mAb (Clone 1B5) | CAC-PRPG-BC-M03 | MS | All Animals |
| Anti 6-Sulfated Unsaturated Disaccharide Neoepitopes (C-6-S "stubs") of Chondroitin Sulfate mAb (Clone 3B3) | CAC-PRPG-BC-M04 | MS | All Animals |
| Anti Chondroitin Sulfate A (Chondroitin-4-Sulfate) mAb (Clone 2H6) | CAC-NU-07-001 | MS | All Animals |
| Anti Aggrecan Core Protein (Chondroitin Sulfate Proteoglycan 1) mAb (Clone 6F4) | CAC-PRPG-AG-M01 | MS | HU BOV |
| Extracellular matrix: Matrix and basement membrane | |||
| Product name (click for order info) | Cat No (click for datasheet) |
Host | Species specificity |
| Anti Laminin/Nidogen complex mAb (Clone 331G3) | CAC-PRPG-NDG-M01 | MS | HU |
| Anti Laminin Subunit Alpha-3 mAb (Clone BM515) | CAC-NU-01-LA3 | MS | HU BOV RAB |
| Anti Laminin Alpha-4 mAb (Clone 652C4) | CAC-PRPG-LA4-M01 | MS | HU |
| Anti Collagen Alpha-1(VII) Chain mAb (Clone BML39) | CAC-NU-01-CO7 | MS | HU BOV RAB POR |
| Anti Collagen Alpha-1(XII) Chain mAb (Clone 378D5) | CAC-PRPG-CO12-M01 | MS | HU Avian BOV |
| Anti Proprotein Convertase Subtilisin/Kexin Type 6 (PACE4) - HomoB domain pAb (Rabbit, Antiserum) | CAC-SK-T01-001 | RAB | HU |
| Anti Proprotein Convertase Subtilisin/Kexin Type 6 (PACE4) - Propeptide pAb (Rabbit, Antiserum) | CAC-SK-T01-002 | RAB | HU |
| Anti Inter-Alpha-Trypsin Inhibitor Heavy Chain H4 (ITIH4) pAb (Brown Norway Rat, Antiserum) | CAC-ICA-TG2-RTP1 | RT | HU RT |
| Anti Inter-Alpha-Trypsin Inhibitor Heavy Chain H4 (ITIH4) pAb (Sprague Dawley Rat, Antiserum) | CAC-ICA-TG2-RTP2 | RT | HU RT |
| Extracellular matrix: Cell adhesion and hemidesmosome-related | |||
| Product name (click for order info) | Cat No (click for datasheet) |
Host | Species specificity |
| Anti Plectin (PCN/PLTN) mAb (Clone PN753) | CAC-NU-01-PLN | MS | HU RT RAB POR |
| Anti Laminin Subunit Gamma-2 mAb (Clone YN557) | CAC-NU-01-LA2 | MS | HU BOV |
| Anti Collagen Alpha-1(XVII) Chain (BP180/BPAG2) mAb (Clone C34) | CAC-NU-01-BP2 | MS | HU |
| Anti Keratin, Type II Cytoskeletal 8 (Keratin-8) mAb (Clone RL273) | CAC-NU-01-KE8 | MS | HU RAB |
| Anti Dystonin (BPAG1/BP230) mAb (Clone 279) | CAC-NU-01-BP1 | MS | HU RT BOV RAB POR |
| Anti Epithelial Cell Adhesion Molecule (EPCAM) mAb (Clone hrk29) | CAC-HT-MAB1 | MS | HU |
| Anti Integrin Alpha-6 (VLA-6) mAb (Clone 537D5) | CAC-PRPG-ITG-M01 | MS | HU |
| Product name | Anti Keratan Sulfate (KS/Keratosulfate) mAb (Clone 373E1) |
| Cat No | CAC-PRPG-KS-M01 |
| Description | Keratan sulfates (KSs) are sulfated polymers of N-acetyllactosamine structured by repeating (1 3)--D-galactose-(1 4)--D-N-acetylglucosamine units, which are generally sulfated at position C6 of the hexosamine and/or galactosamine. They are mostly covalently bound to core proteins of KS-bearing proteoglycans (PGs), but a few non-proteoglycan KS-substituted macromolecules have been described, and their attachment to protein backbones occurs primarily through an N-linkage involving glucosamine binding to an asparagine residue These are referred to type I KSs and are characteristic of the corneal ECM. KS chains may also be bound to proteins through an O-glycosidic linkage between galactosamine and a serine or threonine residue, i.e. referred to as type II KSs and highly represented in articular cartilage ECM. Phosphocan and other KS-containing PGs of the brain may also carry KS chains attached to the core protein through an alternative mannose-serine/threonine linkage. One of the complexities of KSs is the variable degree of chain branching (i.e. bi-antennary in the cornea and more intricate branching in skeletal KS), which together with the variable extent and positioning of the sulfate groups and the relative frequency, linkage and type of capping fucose and/or neuraminic acid residues, creates a spectrum of putative functionally diverse KS moieties. For instance, sialic acid residues may coincidently, or in an alternated fashion be present in an (1-3), (2-3) or (2-6)-linkage, and may or may not associate with (1-3)-linked fucoses. References: Magro, M., et.al., 2003. Proteomic and post-proteomic characterization of keratan sulfate-glycanated isoforms of thyroglobulin and transferrin uniquely elaborated by papillary thyroid carcinoma. Am J. Pathol. 163, 183-196. |
| Host | Rat |
| Species specificity | All |
| Figure 1 | 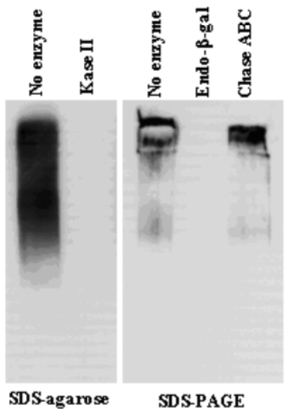 |
| Immunoblot analysis of purified human articular cartilage aggrecan resolved prior to and after keratanase II, endogalactosidase-, or chondroitinase ABC-digestion on SDS-Agarose electrophoresis (left gel) or 3-8% gradient gels. *Band sizing and pattern depends upon the core protein size of the molecule (mostly proteoglycans) bearing keratin sulfate chains. If isolated chains are separated by PAGE, the banding pattern appears as a smear and the approximate molecular weights of the bands depend upon the mass and polydispersity of the chains. |
|
| Figure 2 | 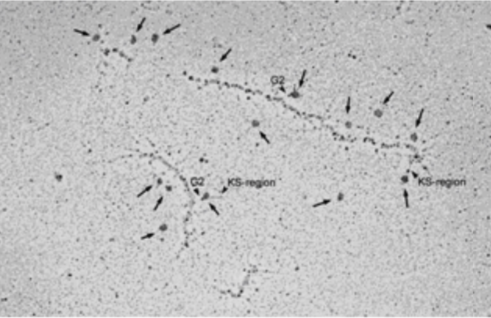 |
| TEM rotary shadowing image of the binding of mAb 373E1 to keratan sulfate chains (arrows) of human articular cartilage aggrecan forming an hyaluronan-proteoglycan aggregate in vitro. | |
| Figure 3 | 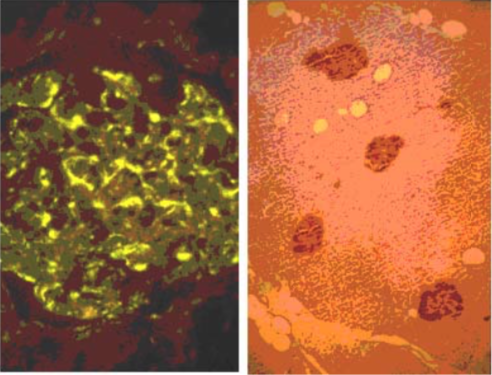 |
| Immunohistochemical staining. (Left) Immunohistochemical staining (FITC-conjugated secondary antibodies) with mAb 373E1 of keratan sulfates of the ECM deposited with a glomerule of human kidney (PFA-OCT embedding and cryosectioning). (Right) Immunohistochemical staining of keratan sulfates deposited within Langerhans islands of human adult pancreas (Formalin-paraffin embedding). |
|
| Product name | Anti Keratan Sulfate (KS/Keratosulfate) mAb (Clone 5D4) |
| Cat No | CAC-PRPG-BC-M01 |
| Description | Keratan sulfate (KS), also called keratosulfate, is any of several sulfated glycosaminoglycans (structural carbohydrates) that are abundant in the cornea, cartilage, and bone. It is also synthesized in the central nervous system where it participates both in development and in the glial scar formation following injury. Keratan sulfates are large, highly hydrated molecules which in joints can act as a cushion to absorb mechanical shock. Like other glycosaminoglycans keratan sulfate is a linear polymer that consists of a repeating disaccharide unit. Keratan sulfate occurs as a proteoglycan (PG) in which KS chains are attached to cell-surface or extracellular matrix proteins, termed core proteins. KS core proteins include lumican, keratocan, mimecan, fibromodulin, PRELP, osteoadherin, and aggrecan. The basic repeating disaccharide unit within keratan sulfate is -3Galβ1-4GlcNAcβ1-. This can be sulfated at carbon position 6 (C6) of either or both the Gal or GlcNAc monosaccharides. However, the detailed primary structure of specific KS types are best considered to be composed of three regions: • Linkage region: the end of the KS chain linked to core proteins. • Repeat region: comprising -3Galβ1-4GlcNAcβ1- repeating disaccharide units. • Chain capping region: the end of the KS chain opposite to the Linkage region. Monoclonal antibody 5D4 recognizes oversulfated heptasaccharide epitopes containing 6-sulfated galactose adjacent with 6-sulfated N-acetyl-glucosamine in oligosaccharide segments of Keratan Sulfate glycosaminoglycan chains. Pre-digestion with Keratanase II removes these epitopes from Keratan Sulfate glycosaminoglycan chains. However, pre-digestion with Keratinase will not necessarily remove these Keratan Sulfate glycosaminoglycan chain epitopes. References: 1) Schwend T, Deaton RJ, Zhang Y, Caterson B & Conrad CW (2012). Corneal sulphated glycosaminoglycans and their effects on trigeminal nerve growth cone behaviour in vitro – roles for ECM in corneal innervation. Invest Opthamol Vis Sci. 53: 2) Caterson B. (2012). Chondroitin sulphate glycosaminoglycans: fun for some and confusion for others. Int. J. of Exp. Path. 93: 1 – 10 PubMed: 22264297 3) Liles M, Palka BP, Harris A,Kerr BC, Hughes CE, Young RD, Meek KM, Caterson B, Quantok AJ (2010). Differential relative sulphation of keratan sulphate glycosaminoglycan in the chick cornea during embryonic development. Invest. Opthalmol. Vis. Sci. 51: 1365-1372 PubMed: 19815728 4) Davies L, Blain E, Caterson B and Duance VC (2008). Chondroitin sulphate impedes the migration of a sub-population of articular cartilage chondrocytes. Osteoarthritis & Cartilage 16: 855 - 864 PubMed: 18222711 5) Hayes AJ, Hughes CE & Caterson B (2008). Antibodies and immunohistochemistry in extracellular matrix research. Methods 45: 10 – 21 PubMed: 18442701 6) Hayes AJ, Hall A, Brown L, Tubo R & Caterson B (2007). Macromolecular organization and in vitro growth characteristics of scaffold-free neocartilage grafts. J. Histochem. Cytochem. 55: 853 – 866. PubMed: 17478447 7) Caterson B, Mahmoodian F, Sorrell JM, Hardingham TE, Bayliss MT, Carney SL, Ratcliffe A & Muir H (1990). Modulation of native chondroitin sulfate structure in tissue development and in disease. J. Cell Sci. 97: 411 – 417. PubMed: 1705939 8) Mehmet H, Scudder P, Tang, PW, Hounsell, EF, Caterson, B & Feizi T (1986). Antigenic determinants recognized by three monoclonal antibodies to keratan sulfate involve sulfated hepta- or larger oligosaccharides of the poly-N-acetyllactosamine series. Eur. J. Biochem. 157: 385 – 391. PubMed: 2423332 9) Funderburgh JL, Caterson B & Conrad GW (1986). Keratan sulfate proteoglycan during embryonic development of the chicken cornea. Developmental Biology 116: 267 – 277 PubMed: 2942429 10) Katz H, Austen KF, Caterson B, & Stevens RL (1986). Secretory granules of Heparin-containing Rat serosal mast cells also possess highly sulfated chondroitin sulfate proteoglycans. J. Biol. Chem. 261: 13393 - 13396 PubMed: 3531203 11) Caterson B, Christner JE, Baker JR & Couchman JR (1985). The production and characterization of monoclonal antibodies directed against connective tissue proteoglycans. Federation Proceedings 44: 386 – 393. PubMed: 257841 12) Couchman JR, Caterson B, Christner JE & Baker JR (1984). Mapping by monoclonal antibody detection of glycosaminoglycans in connective tissues. Nature 307: 650 – 652. PubMed: 6420711 13) Caterson B, Christner JE & Baker JR (1983). Identification of a monoclonal antibody that specifically recognizes corneal and skeletal keratan sulfate. Monoclonal antibodies to cartilage proteoglycan. J. Biol. Chem. 258: 8848 – 8854 PubMed: 6223038 |
| Host | Mouse |
| Species specificity | All |
| Product name | Anti 4-Sulfated Unsaturated Disaccharide Neoepitopes (C-4-S "stubs") of Chondroitin Sulfate or Dermatan Sulfate mAb (Clone 2B6) |
| Cat No | CAC-PRPG-BC-M02 |
| Description | Monoclonal antibody 2B6 recognizes 4-sulfated unsaturated disaccharide neoepitopes (i.e. C-4-S "stubs") generated at the non-reducing terminal of Chondroitin Sulfate or Dermatan Sulfate glycosaminoglycan chains that have been pre-digested with Chondroitinase ABC [see Figure 2; Caterson B (2012) Int. J. Exp. Pathol. 93: 1 - 10] but only Chondroitin Sulfate glycosaminoglycan chains pre-digested with Chondroitinase ACII or only Dermatan Sulfate glycosaminoglycan chains pre-digested with Chondroitinase B. References: 1) Schwend T, Deaton RJ, Zhang Y, Caterson B & Conrad CW (2012). Corneal sulphated glycosaminoglycans and their effects on trigeminal nerve growth cone behaviour in vitro – roles for ECM in corneal innervation. Invest Opthamol Vis Sci. 53: 2) Caterson B. (2012). Chondroitin sulphate glycosaminoglycans: fun for some and confusion for others. Int. J. of Exp. Path. 93: 1 – 10 PubMed: 22264297 3) Liles M, Palka BP, Harris A,Kerr BC, Hughes CE, Young RD, Meek KM, Caterson B, Quantok AJ (2010). Differential relative sulphation of keratan sulphate glycosaminoglycan in the chick cornea during embryonic development. Invest. Opthalmol. Vis. Sci. 51: 1365-1372 PubMed: 19815728 4) Davies L, Blain E, Caterson B and Duance VC (2008). Chondroitin sulphate impedes the migration of a sub-population of articular cartilage chondrocytes. Osteoarthritis & Cartilage 16: 855 - 864 PubMed: 18222711 5) Hayes AJ, Hughes CE & Caterson B (2008). Antibodies and immunohistochemistry in extracellular matrix research. Methods 45: 10 – 21 PubMed: 18442701 6) Hayes AJ, Hall A, Brown L, Tubo R & Caterson B (2007). Macromolecular organization and in vitro growth characteristics of scaffold-free neocartilage grafts. J. Histochem. Cytochem. 55: 853 – 866. PubMed: 17478447 7) Caterson B, Mahmoodian F, Sorrell JM, Hardingham TE, Bayliss MT, Carney SL, Ratcliffe A & Muir H (1990). Modulation of native chondroitin sulfate structure in tissue development and in disease. J. Cell Sci. 97: 411 – 417. PubMed: 1705939 8) Mehmet H, Scudder P, Tang, PW, Hounsell, EF, Caterson, B & Feizi T (1986). Antigenic determinants recognized by three monoclonal antibodies to keratan sulfate involve sulfated hepta- or larger oligosaccharides of the poly-N-acetyllactosamine series. Eur. J. Biochem. 157: 385 – 391. PubMed: 2423332 9) Funderburgh JL, Caterson B & Conrad GW (1986). Keratan sulfate proteoglycan during embryonic development of the chicken cornea. Developmental Biology 116: 267 – 277 PubMed: 2942429 10) Katz H, Austen KF, Caterson B, & Stevens RL (1986). Secretory granules of Heparin-containing Rat serosal mast cells also possess highly sulfated chondroitin sulfate proteoglycans. J. Biol. Chem. 261: 13393 - 13396 PubMed: 3531203 11) Caterson B, Christner JE, Baker JR & Couchman JR (1985). The production and characterization of monoclonal antibodies directed against connective tissue proteoglycans. Federation Proceedings 44: 386 – 393. PubMed: 257841 12) Couchman JR, Caterson B, Christner JE & Baker JR (1984). Mapping by monoclonal antibody detection of glycosaminoglycans in connective tissues. Nature 307: 650 – 652. PubMed: 6420711 13) Caterson B, Christner JE & Baker JR (1983). Identification of a monoclonal antibody that specifically recognizes corneal and skeletal keratan sulfate. Monoclonal antibodies to cartilage proteoglycan. J. Biol. Chem. 258: 8848 – 8854 PubMed: 6223038 |
| Host | Mouse |
| Species specificity | All |
| Figure 1 | 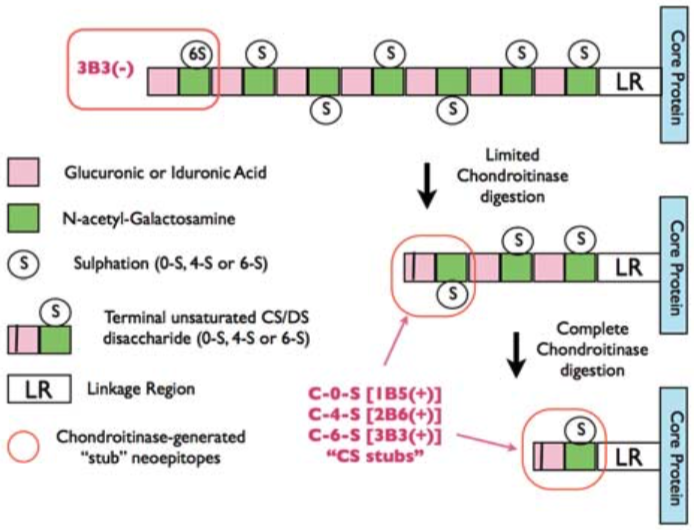 |
| Epitope mapping scheme of chondroitin sulfate-detecting antibody clones 1B5, 2B6 and 3B3. | |
| Figure 2 | 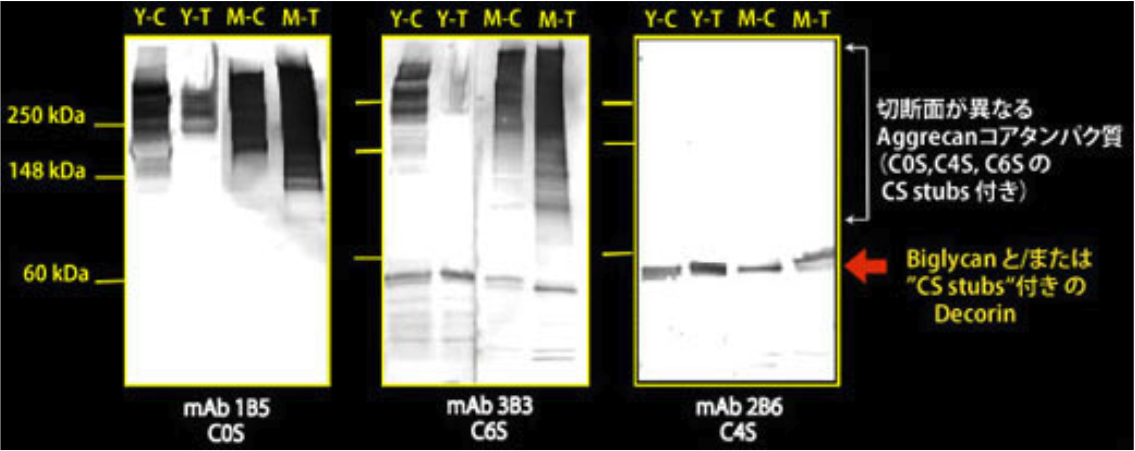 |
| Immunoblot analysis of chondroitin sulfate detection antibodies 1B5, 2B6 and 3B3 using HCl extract of bovine tendon. Age (Y: young, M: mature) State of tendon (C: compressed, T: tensioned) |
|
| Product name | Anti Unsulfated Unsaturated Disaccharide Neoepitopes (C-0-S "stubs") of Chondroitin Sulfate mAb (Clone 1B5) |
| Cat No | CAC-PRPG-BC-M03 |
| Description | Monoclonal antibody 1B5 recognizes unsulfated unsaturated disaccharide neoepitopes (i.e. C-0-S "stubs") generated at the non-reducing terminal of Chondroitin Sulfate glycosaminoglycan chains that have been pre-digested with either Chondroitinase ABC or Chondroitinase ACII [see Figure 2; Caterson B (2012) Int. J. Exp. Pathol. 93: 1 - 10]. References: 1) Schwend T, Deaton RJ, Zhang Y, Caterson B & Conrad CW (2012). Corneal sulphated glycosaminoglycans and their effects on trigeminal nerve growth cone behaviour in vitro – roles for ECM in corneal innervation. Invest Opthamol Vis Sci. 53: 2) Caterson B. (2012). Chondroitin sulphate glycosaminoglycans: fun for some and confusion for others. Int. J. of Exp. Path. 93: 1 – 10 PubMed: 22264297 3) Liles M, Palka BP, Harris A,Kerr BC, Hughes CE, Young RD, Meek KM, Caterson B, Quantok AJ (2010). Differential relative sulphation of keratan sulphate glycosaminoglycan in the chick cornea during embryonic development. Invest. Opthalmol. Vis. Sci. 51: 1365-1372 PubMed: 19815728 4) Davies L, Blain E, Caterson B and Duance VC (2008). Chondroitin sulphate impedes the migration of a sub-population of articular cartilage chondrocytes. Osteoarthritis & Cartilage 16: 855 - 864 PubMed: 18222711 5) Hayes AJ, Hughes CE & Caterson B (2008). Antibodies and immunohistochemistry in extracellular matrix research. Methods 45: 10 – 21 PubMed: 18442701 6) Hayes AJ, Hall A, Brown L, Tubo R & Caterson B (2007). Macromolecular organization and in vitro growth characteristics of scaffold-free neocartilage grafts. J. Histochem. Cytochem. 55: 853 – 866. PubMed: 17478447 7) Caterson B, Mahmoodian F, Sorrell JM, Hardingham TE, Bayliss MT, Carney SL, Ratcliffe A & Muir H (1990). Modulation of native chondroitin sulfate structure in tissue development and in disease. J. Cell Sci. 97: 411 – 417. PubMed: 1705939 8) Mehmet H, Scudder P, Tang, PW, Hounsell, EF, Caterson, B & Feizi T (1986). Antigenic determinants recognized by three monoclonal antibodies to keratan sulfate involve sulfated hepta- or larger oligosaccharides of the poly-N-acetyllactosamine series. Eur. J. Biochem. 157: 385 – 391. PubMed: 2423332 9) Funderburgh JL, Caterson B & Conrad GW (1986). Keratan sulfate proteoglycan during embryonic development of the chicken cornea. Developmental Biology 116: 267 – 277 PubMed: 2942429 10) Katz H, Austen KF, Caterson B, & Stevens RL (1986). Secretory granules of Heparin-containing Rat serosal mast cells also possess highly sulfated chondroitin sulfate proteoglycans. J. Biol. Chem. 261: 13393 - 13396 PubMed: 3531203 11) Caterson B, Christner JE, Baker JR & Couchman JR (1985). The production and characterization of monoclonal antibodies directed against connective tissue proteoglycans. Federation Proceedings 44: 386 – 393. PubMed: 257841 12) Couchman JR, Caterson B, Christner JE & Baker JR (1984). Mapping by monoclonal antibody detection of glycosaminoglycans in connective tissues. Nature 307: 650 – 652. PubMed: 6420711 13) Caterson B, Christner JE & Baker JR (1983). Identification of a monoclonal antibody that specifically recognizes corneal and skeletal keratan sulfate. Monoclonal antibodies to cartilage proteoglycan. J. Biol. Chem. 258: 8848 – 8854 PubMed: 6223038 |
| Host | Mouse |
| Species specificity | All |
| Figure 1 | 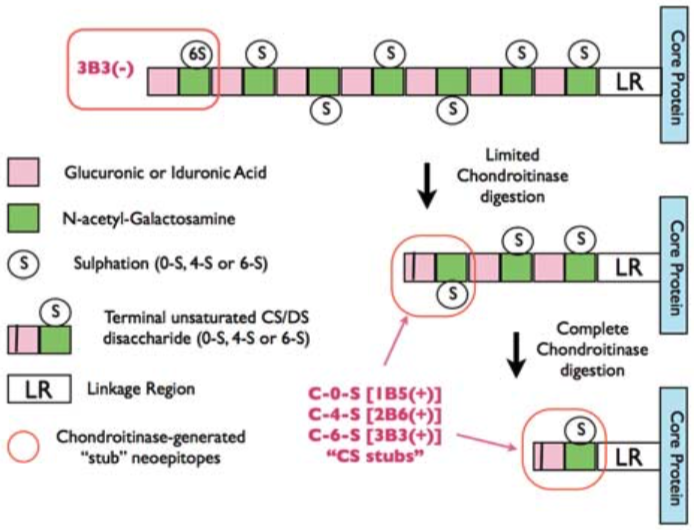 |
| Epitope mapping scheme of chondroitin sulfate-detecting antibody clones 1B5, 2B6 and 3B3. | |
| Figure 2 | 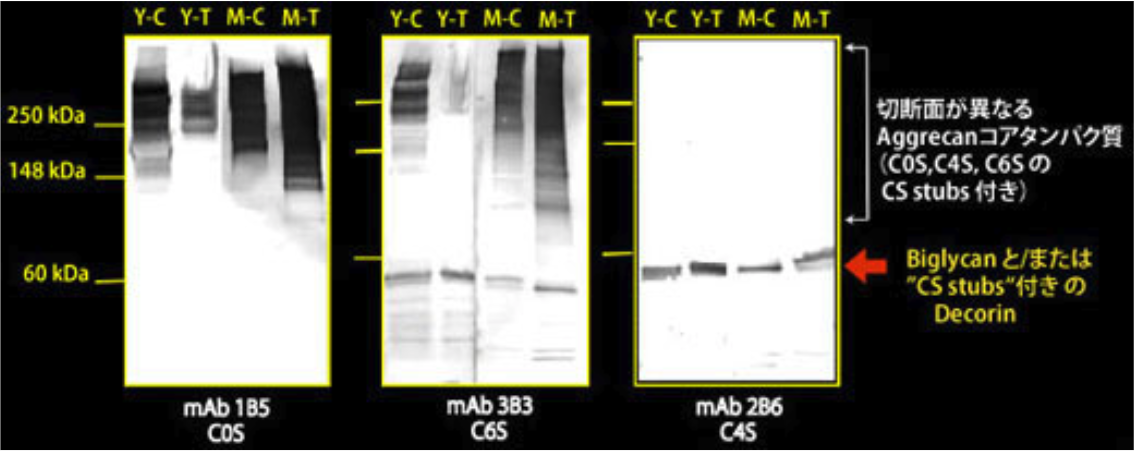 |
| Immunoblot analysis of chondroitin sulfate detection antibodies 1B5, 2B6 and 3B3 using HCl extract of bovine tendon. Age (Y: young, M: mature) State of tendon (C: compressed, T: tensioned) |
|
| Product name | Anti 6-Sulfated Unsaturated Disaccharide Neoepitopes (C-6-S "stubs") of Chondroitin Sulfate mAb (Clone 3B3) |
| Cat No | CAC-PRPG-BC-M04 |
| Description | Monoclonal antibody 3B3 recognizes 6-sulfated unsaturated disaccharide neoepitopes (i.e. C-6-S "stubs") generated at the non-reducing terminal of Chondroitin Sulfate glycosaminoglycan chains that have been pre-digested with either Chondroitinase ABC or Chondroitinase ACII. Monoclonal antibody 3B3 also recognizes a non-reducing end saturated disaccharide epitope in 'native' Chondroitin Sulfate glycosaminoglycan chains consisting of a terminal glucuronic acid adjacent to 6-sulfated N-acetyl-galactosamine. The chondroitinase-generated neoepitope is often denoted as 3B3(+) and the 'native' terminal epitope as 3B3(-) in publications [see Figure 2; Caterson B (2012) Int. J. Exp. Pathol. 93: 1 - 10]. References: 1) Schwend T, Deaton RJ, Zhang Y, Caterson B & Conrad CW (2012). Corneal sulphated glycosaminoglycans and their effects on trigeminal nerve growth cone behaviour in vitro – roles for ECM in corneal innervation. Invest Opthamol Vis Sci. 53: 2) Caterson B. (2012). Chondroitin sulphate glycosaminoglycans: fun for some and confusion for others. Int. J. of Exp. Path. 93: 1 – 10 PubMed: 22264297 3) Liles M, Palka BP, Harris A,Kerr BC, Hughes CE, Young RD, Meek KM, Caterson B, Quantok AJ (2010). Differential relative sulphation of keratan sulphate glycosaminoglycan in the chick cornea during embryonic development. Invest. Opthalmol. Vis. Sci. 51: 1365-1372 PubMed: 19815728 4) Davies L, Blain E, Caterson B and Duance VC (2008). Chondroitin sulphate impedes the migration of a sub-population of articular cartilage chondrocytes. Osteoarthritis & Cartilage 16: 855 - 864 PubMed: 18222711 5) Hayes AJ, Hughes CE & Caterson B (2008). Antibodies and immunohistochemistry in extracellular matrix research. Methods 45: 10 – 21 PubMed: 18442701 6) Hayes AJ, Hall A, Brown L, Tubo R & Caterson B (2007). Macromolecular organization and in vitro growth characteristics of scaffold-free neocartilage grafts. J. Histochem. Cytochem. 55: 853 – 866. PubMed: 17478447 7) Caterson B, Mahmoodian F, Sorrell JM, Hardingham TE, Bayliss MT, Carney SL, Ratcliffe A & Muir H (1990). Modulation of native chondroitin sulfate structure in tissue development and in disease. J. Cell Sci. 97: 411 – 417. PubMed: 1705939 8) Mehmet H, Scudder P, Tang, PW, Hounsell, EF, Caterson, B & Feizi T (1986). Antigenic determinants recognized by three monoclonal antibodies to keratan sulfate involve sulfated hepta- or larger oligosaccharides of the poly-N-acetyllactosamine series. Eur. J. Biochem. 157: 385 – 391. PubMed: 2423332 9) Funderburgh JL, Caterson B & Conrad GW (1986). Keratan sulfate proteoglycan during embryonic development of the chicken cornea. Developmental Biology 116: 267 – 277 PubMed: 2942429 10) Katz H, Austen KF, Caterson B, & Stevens RL (1986). Secretory granules of Heparin-containing Rat serosal mast cells also possess highly sulfated chondroitin sulfate proteoglycans. J. Biol. Chem. 261: 13393 - 13396 PubMed: 3531203 11) Caterson B, Christner JE, Baker JR & Couchman JR (1985). The production and characterization of monoclonal antibodies directed against connective tissue proteoglycans. Federation Proceedings 44: 386 – 393. PubMed: 257841 12) Couchman JR, Caterson B, Christner JE & Baker JR (1984). Mapping by monoclonal antibody detection of glycosaminoglycans in connective tissues. Nature 307: 650 – 652. PubMed: 6420711 13) Caterson B, Christner JE & Baker JR (1983). Identification of a monoclonal antibody that specifically recognizes corneal and skeletal keratan sulfate. Monoclonal antibodies to cartilage proteoglycan. J. Biol. Chem. 258: 8848 – 8854 PubMed: 6223038 |
| Host | Mouse |
| Species specificity | All |
| Figure 1 |  |
| Epitope mapping scheme of chondroitin sulfate-detecting antibody clones 1B5, 2B6 and 3B3. | |
| Figure 2 | 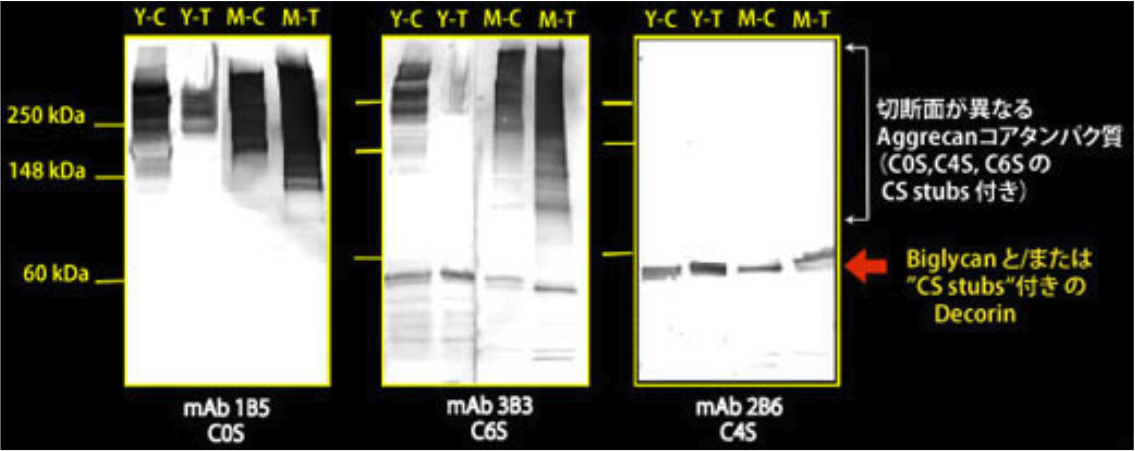 |
| Immunoblot analysis of chondroitin sulfate detection antibodies 1B5, 2B6 and 3B3 using HCl extract of bovine tendon. Age (Y: young, M: mature) State of tendon (C: compressed, T: tensioned) |
|
| Product name | Anti Chondroitin Sulfate A (Chondroitin-4-Sulfate) mAb (Clone 2H6) |
| Cat No | CAC-NU-07-001 |
| Description | Chondroitin sulfate, a polysaccharide moiety of proteoglycans, is one of the major components of the extracellular matrix. It is composed of the repeating unit, [→4GlcA 1→3GalNAc 1→], commonly sulfated at C-4 and/or C-6 of GalNAc. In the central nervous system, the most abundant glycosaminoglycan is chondroitin sulfate rich in [GlcA-GalNAc(4S)], namely chondroitin sulfate A. Chondroitin sulfate polysaccharides are involved in the formation, regeneration, and maintenance of the neural network. This monoclonal antibody effectively recognizes chondroitin sulfate A, especially in glycosaminoglycans occurring in the developing central nervous system. References: 1) Oohira, A., et al. (1994) Neuroscience. 60:145-157. PMID: 8052408. 2) Yamamoto, Y., et al. (1995) Eur. J. Histochem. 39:265-272. PMID: 8835180. |
| Host | MS |
| Species specificity | Animal |
| Figure 1 | 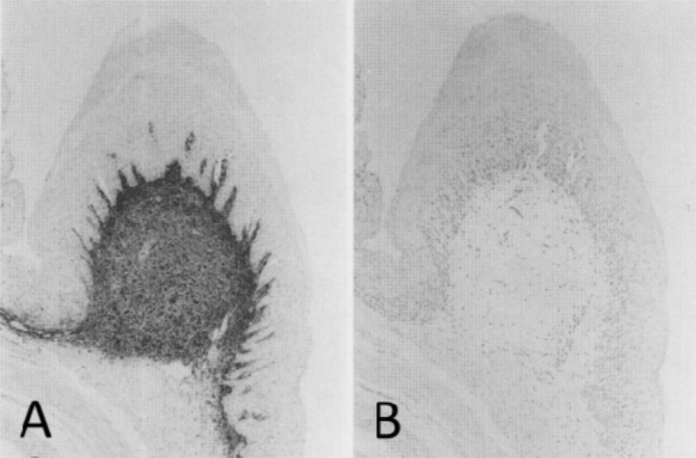 |
| Immunohistochemical staining using monoclonal anti-chondroitin sulfate A antibody (clone 2H6). Paraffin sections of omasal papilla from adult sheep were stained with monoclonal anti-chondroitin sulfate A antibody before (A) and after (B) digestion with chondroitinase ABC. After pretreatment with the enzyme, the immunoreactivity completely disappeared. [Reference: Eur. J. Histochem. (1995) 39:265-272.] | |
| Proteoglycans |
|
| Except for hyaluronan, all glysocaminoglycans (GAGs) are found covalently attached to proteins in the form of proteoglycans, which are made by most animal cells. The polypeptide chain, or core protein, of a proteoglycan is made on membrane-bound ribosomes and threaded into the lumen of the endoplasmic reticulum. The polysaccharide chains are mainly assembled on this core protein in the Golgi apparatus. First, a special link tetrasaccharide is attached to a serine side chain on the core protein to serve as a primer for polysaccharide growth; then, one sugar at a time is added by specific glycosyl transferases. While still in the Golgi apparatus, many of the polymerized sugars are covalently modified by a sequential and coordinated series of reactions. Epimerizations alter the configuration of the substituents around individual carbon atoms in the sugar molecule; sulfations increase the negative charge. Proteoglycans are usually easily distinguished from other glycoproteins by the nature, quantity, and arrangement of their sugar side chains. By definition, at least one of the sugar side chains of a proteoglycan must be a GAG. Whereas glycoproteins contain 1–60% carbohydrate by weight in the form of numerous relatively short, branched oligosaccharide chains, proteoglycans can contain as much as 95% carbohydrate by weight, mostly in the form of long, unbranched GAG chains, each typically about 80 sugars long. Proteoglycans can be huge. The proteoglycan aggrecan, for example, which is a major component of cartilage, has a mass of about 3 × 106 daltons with over 100 GAG chains. Other proteoglycans are much smaller and have only 1–10 GAG chains; an example is decorin, which is secreted by fibroblasts and has a single GAG chain. In principle, proteoglycans have the potential for almost limitless heterogeneity. Even a single type of core protein can vary greatly in the number and types of attached GAG chains. Moreover, the underlying repeating pattern of disaccharides in each GAG can be modified by a complex pattern of sulfate groups. The heterogeneity of these GAGs makes it difficult to identify and classify proteoglycans in terms of their sugars. The sequences of many core proteins have been determined with the aid of recombinant DNA techniques, and they, too, are extremely diverse. Although a few small families have been recognized, no common structural feature clearly distinguishes proteoglycan core proteins from other proteins, and many have one or more domains that are homologous to domains found in other proteins of the extracellular matrix or plasma membrane. Thus, it is probably best to regard proteoglycans as a diverse group of highly glycosylated glycoproteins whose functions are mediated by both their core proteins and their GAG chains. Given the great abundance and structural diversity of proteoglycan molecules, it would be surprising if their function were limited to providing hydrated space around and between cells. Their GAG chains, for example, can form gels of varying pore size and charge density; one possible function, therefore, is to serve as selective sieves to regulate the traffic of molecules and cells according to their size, charge, or both. Evidence suggests that a heparan sulfate proteoglycan called perlecan has this role in the basal lamina of the kidney glomerulus, which filters molecules passing into the urine from the bloodstream (discussed below). Proteoglycans are thought to have a major role in chemical signaling between cells. They bind various secreted signal molecules, such as certain protein growth factors, and can enhance or inhibit their signaling activity. For example, the heparan sulfate chains of proteoglycans bind to fibroblast growth factors (FGFs), which stimulate a variety of cell types to proliferate; this interaction oligomerizes the growth factor molecules, enabling them to cross-link and activate their cell-surface receptors, which are transmembrane tyrosine kinases. Whereas in most cases the signal molecules bind to the GAG chains of the proteoglycan, this is not always so. Some members of the transforming growth factor β (TGF-β) family bind to the core proteins of several matrix proteoglycans, including decorin; binding to decorin inhibits the activity of the growth factors. Proteoglycans also bind, and regulate the activities of, other types of secreted proteins, including proteolytic enzymes (proteases) and protease inhibitors. Binding to a proteoglycan could control the activity of a secreted protein in any of the following ways: (1) it could immobilize the protein close to the site where it is produced, thereby restricting its range of action; (2) it could sterically block the activity of the protein; (3) it could provide a reservoir of the protein for delayed release; (4) it could protect the protein from proteolytic degradation, thereby prolonging its action; (5) it could alter or concentrate the protein for more effective presentation to cell-surface receptors. Proteoglycans are thought to act in all these ways to help regulate the activities of secreted proteins. An example of the last function occurs in inflammatory responses, in which heparan sulfate proteoglycans immobilize secreted chemotactic attractants called chemokines on the endothelial surface of a blood vessel at an inflammatory site. In this way, the chemokines remain there for a prolonged period, stimulating white blood cells to leave the bloodstream and migrate into the inflamed tissue. [Adapted from: Alberts B, Johnson A, Lewis J, et al. Molecular Biology of the Cell. 4th edition. New York: Garland Science; 2002. The Extracellular Matrix of Animals. Available from: https://www.ncbi.nlm.nih.gov/books/NBK26810/] |
|
| Product name | Anti Aggrecan Core Protein (Chondroitin Sulfate Proteoglycan 1) mAb (Clone 6F4) |
| Cat No | CAC-PRPG-AG-M01 |
| Description | Aggrecan is the major proteoglycan in the articular cartilage (synthesized by mature chondrocytes) and in perineuronal nets of the CNS. While its precise function around CNS neurons remains obscure, in articular cartilage it contributes to creating the hydrated gel structure of the ECM via its interaction with hyaluronan, link protein, CMPs, COMP and collagen type IX. Deletion of the aggrecan gene causes early disturbances in chondrogenesis and brain defects. Aggrecan is a multimodular molecule whose core protein is composed of three globular domains denoted G1, G2, and G3, a large extended region spanning the portion of the molecule between the globular domains G1 and G2 and containing the majority of the GAG attachment sites and a second GAG-bearing inter-globular domain (IGD) occurs between G2 and G3. The GAG attachment domain between G1 and G2 contains mainly chondroitin sulphate chains (up to 40) and some keratan sulfate chains. The inter-globular G2-G3 domain exclusively bears keratan sulphate chains. The corresponding core protein region of sclera and brain aggrecans do not seem to contain keratan sulphates. The G1 amino-terminal domain of the aggrecan core protein has the same structural motif as link protein and is responsible for the binding of the proteoglycan to hyaluronan and link protein. The G2 globular domain is homologous to the tandem repeats of G1 and of link protein and is crucial for the synthesis and cellular secretion of aggrecan. The G3 globular domain makes up the carboxyl terminus of the core protein and is similarly responsible for post-translational processing of the proteoglycan and its secretion, as well as for its molecular interactions with other cartilage ECM components. Fully glycosylated/glycanated aggrecan of articular cartilage has an average size of 2,400-2,500 kDa, but its Mr may vary with age and the conditions of the cartilage tissue. The non-glycosylated/non-glycanated core protein has an approximate Mr of 240 kDa. References: Virgintino D, et all., (2009) Aggrecan isoforms of perineuronal nets identify subsets of parvalbumin and calbindin neurons differentially distributed in cortical layers II-VI of human adult cortex. J. Cell. Mol. Medicine 13, 3151-3173.I161:I164. |
| Host | Mouse |
| Species specificity | HU BOV |
| Figure 1 | 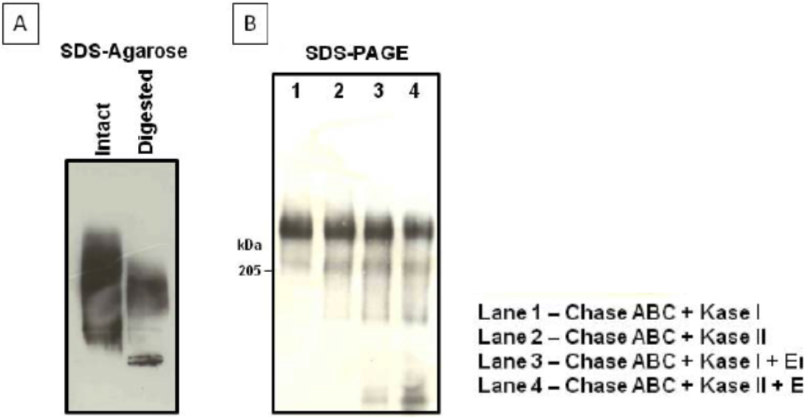 |
| Immunoblot analysis of purified human cartilage aggrecan. (A) SDS-agarose gel electrophoresis (0.5%) before and after combined chondroitinase ABC digestion of aggrecan. (B) SDS-PAGE on 3-8% linear gradient gels, after the combined digestion of aggrecan indicated in the legend. |
|
| Figure 2 | 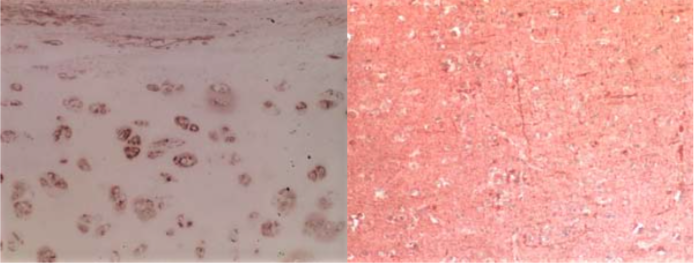 |
| Immunohistochemical analysis of normal human articular cartilage (left) and brain cortex (right). | |
| Product name | Anti Aggrecan Core Protein (Chondroitin Sulfate Proteoglycan 1) mAb (Clone 5D3) |
| Cat No | CAC-PRPG-AG-M02 |
| Description | Aggrecan is the major proteoglycan in the articular cartilage (synthesized by mature chondrocytes) and in perineuronal nets of the CNS. While its precise function around CNS neurons remains obscure, in articular cartilage it contributes to creating the hydrated gel structure of the ECM via its interaction with hyaluronan, link protein, CMPs, COMP and collagen type IX. Deletion of the aggrecan gene causes early disturbances in chondrogenesis and brain defects. Aggrecan is a multimodular molecule whose core protein is composed of three globular domains denoted G1, G2, and G3, a large extended region spanning the portion of the molecule between the globular domains G1 and G2 and containing the majority of the GAG attachment sites and a second GAG-bearing inter-globular domain (IGD) occurs between G2 and G3. The GAG attachment domain between G1 and G2 contains mainly chondroitin sulphate chains (up to 40) and some keratan sulfate chains. The inter-globular G2-G3 domain exclusively bears keratan sulphate chains. The corresponding core protein region of sclera and brain aggrecans do not seem to contain keratan sulphates. The G1 amino-terminal domain of the aggrecan core protein has the same structural motif as link protein and is responsible for the binding of the proteoglycan to hyaluronan and link protein. The G2 globular domain is homologous to the tandem repeats of G1 and of link protein and is crucial for the synthesis and cellular secretion of aggrecan. The G3 globular domain makes up the carboxyl terminus of the core protein and is similarly responsible for post-translational processing of the proteoglycan and its secretion, as well as for its molecular interactions with other cartilage ECM components. Fully glycosylated/glycanated aggrecan of articular cartilage has an average size of 2,400-2,500 kDa, but its Mr may vary with age and the conditions of the cartilage tissue. The non-glycosylated/non-glycanated core protein has an approximate Mr of 240 kDa. References: Virgintino D, et all., (2009) Aggrecan isoforms of perineuronal nets identify subsets of parvalbumin and calbindin neurons differentially distributed in cortical layers II-VI of human adult cortex. J. Cell. Mol. Medicine 13, 3151-3173.I161:I164. |
| Host | Mouse |
| Species specificity | HU BOV |
| Figure 1 | 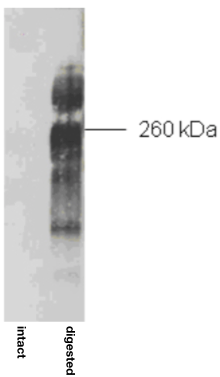 |
| Immunoblot analysis of purified human articular cartilage aggrecan prior to (intact) and after combined keratanase I, endo-galactosidase and chondroitinase ABC-digestion. SDS-PAGE on 3-8% linear gradient gels under reducing conditions | |
| Figure 2 | 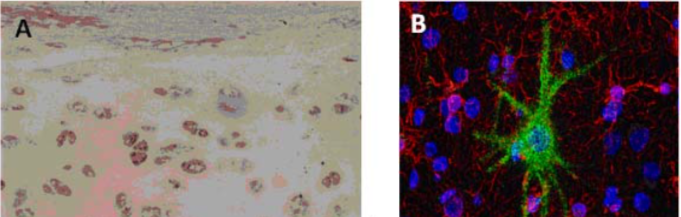 |
| (A) Immunostaining of human articular cartilage. (B) Double immunostaining for aggrecan and neurofilaments in human adult cerebral cortex. Cell nuclei are counterstained with TO-PRO-1. | |
| Product name | Anti Aggrecan Core Protein (Chondroitin Sulfate Proteoglycan 1) mAb (Clone 5G2) |
| Cat No | CAC-PRPG-AG-M03 |
| Description | Aggrecan is the major proteoglycan in the articular cartilage (synthesized by mature chondrocytes) and in perineuronal nets of the CNS. While its precise function around CNS neurons remains obscure, in articular cartilage it contributes to creating the hydrated gel structure of the ECM via its interaction with hyaluronan, link protein, CMPs, COMP and collagen type IX. Deletion of the aggrecan gene causes early disturbances in chondrogenesis and brain defects. Aggrecan is a multimodular molecule whose core protein is composed of three globular domains denoted G1, G2, and G3, a large extended region spanning the portion of the molecule between the globular domains G1 and G2 and containing the majority of the GAG attachment sites and a second GAG-bearing inter-globular domain (IGD) occurs between G2 and G3. The GAG attachment domain between G1 and G2 contains mainly chondroitin sulphate chains (up to 40) and some keratan sulfate chains. The inter-globular G2-G3 domain exclusively bears keratan sulphate chains. The corresponding core protein region of sclera and brain aggrecans do not seem to contain keratan sulphates. The G1 amino-terminal domain of the aggrecan core protein has the same structural motif as link protein and is responsible for the binding of the proteoglycan to hyaluronan and link protein. The G2 globular domain is homologous to the tandem repeats of G1 and of link protein and is crucial for the synthesis and cellular secretion of aggrecan. The G3 globular domain makes up the carboxyl terminus of the core protein and is similarly responsible for post-translational processing of the proteoglycan and its secretion, as well as for its molecular interactions with other cartilage ECM components. Fully glycosylated/glycanated aggrecan of articular cartilage has an average size of 2,400-2,500 kDa, but its Mr may vary with age and the conditions of the cartilage tissue. The non-glycosylated/non-glycanated core protein has an approximate Mr of 240 kDa. References: Virgintino D, et all., (2009) Aggrecan isoforms of perineuronal nets identify subsets of parvalbumin and calbindin neurons differentially distributed in cortical layers II-VI of human adult cortex. J. Cell. Mol. Medicine 13, 3151-3173.I161:I164. |
| Host | Mouse |
| Species specificity | HU |
| Figure 1 | 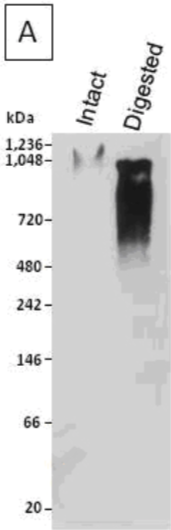 |
| Immunoblot analysis of a human articular cartilage aggrecan preparation following SDS-PAGE on 3-8% linear gradient gels under reducing conditions, prior to (Intact) and after combined chondroitinase ABC and keratanase II digestion (Digested). | |
| Figure 2 |  |
| Immunostaining for aggrecan in human articular cartilage (B) and aggrecan in human adult cerebral cortex (arrows point to neurons and perineuronal nets) (C). | |
| Product name | Anti Biglycan (Bone/cartilage Proteoglycan I) mAb (Clone 905A7) |
| Cat No | CAC-PRPG-BG-M01 |
| Description | Biglycan is a small secreted ECM proteoglycan belonging to the small leucine-rich repeat (SLRP) subfamily. It contains a central 12 LRR domain flanked by small cysteine clusters at either side. The structure of biglycan core protein is highly conserved across species; over 90% has been reported for rat, mouse, bovine and human biglycan core proteins. Two glycosaminoglycan chains of the chondroitin or dermatan sulfate type are attached near the amino terminus of the core protein, generating a molecule that in its fully glycanated form reaches 250 kDa. Deposition of non-glycanated forms of biglycan have been shown to increase in cartilage and bone ECMs with age. Like decorin and fibromodulin, biglycan controls collagen fibrillogenesis and, partly through this action, it is believed to play a key role in bone mineralization and the assembly of cartilage and corneal ECM. In fact, deletion of the biglycan gene leads to an osteoporosis-like phenotype and double knockout of biglycan and fibromodulin cause severe cartilage and macular degeneration. The biglycan core protein binds BMP4 and may influence its bioactivity, especially in the context of osteoblast differentiation/maturation. This is believed to depend upon the ability of biglycan to regulate the interaction of the growth factor with its extracellular antagonist chordin. There is also evidence that biglycan may bind TGFb1 and affect homeostasis and new formation of blood vessels. Furthermore, BGN may affect signal transduction during cell growth and differentiation via induction of the cyclin-dependent kinase inhibitor p27KIP1. Biglycan-induced activation of RhoA and Rac1 signaling increases migration of lung fibroblasts. Moreover, adenovirus-mediated gene transfer of BGN induced a fibroblastic response in the lung, indicating a role of BGN in fibrogenesis. Finally, biglycan is up-regulated during inflammation and augments this condition by contributing to Toll-like receptors 2 and 4 signaling in macrophages. |
| Host | Mouse |
| Species specificity | HU BOV |
| Figure 1 | 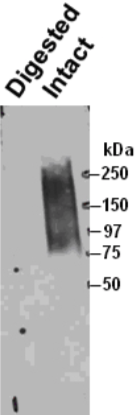 |
| Immunoprecipitation of biglycan from human smooth muscle cells and Western blotting with a second anti-biglycan mAb on the intact (right) and chondroitinase ABC predigested (left) precipitate resolved by SDS-PAGE on gradient 4-18% gels. | |
| Figure 2 | 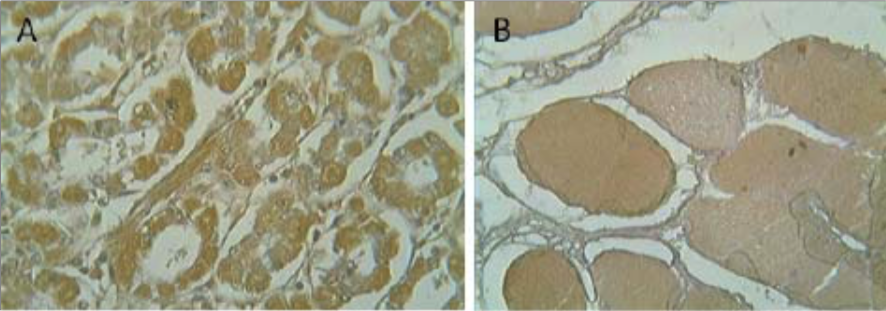 |
| Immunohistochemistry on (A) human intestine and (B) human skeletal muscle. | |
| Product name | Anti Decorin (Bone Proteoglycan II) mAb (Clone 889C7) |
| Cat No | CAC-PRPG-DC-M01 |
| Description | Decorin is a ubiquitous small ECM proteoglycan that is closely related in structure to, biglycan, and which belongs to the small leucine-rich proteoglycan (SLRP) subfamily. Its core protein may be found frequently associated with the cell surface and normally carries a single chondroitin sulfate or dermatan sulfate chain. Its molecular mass in fully glycosylated/glycanated form varies from 90-240 kDa, while its unglycosylated/unglycanated core protein has a Mr of about 45 kDa. Decorin interacts with several ECM components, including fibrillar collagens, fibronectin, thrombospondin and C1q and plays a role in collagen fibrillogenesis. Decorin is upregulated in cancer, inflammed and degenerating tissues, and is critically involved in wound-healing. Infusion of decorin into experimental rodent spinal cord injuries has been shown to suppress scar formation and promote axon growth. Decorin affects various types of cancer by down-regulating the activity of several receptors involved in cell growth and survival. Decorin binds to and modulates the signaling of the epidermal growth factor receptor and other members of the ErbB family of receptor tyrosine kinases. It exerts its antitumor activity by a dual mechanism: via inhibition of these key receptors through their physical downregulation coupled with attenuation of their signaling, and by binding to and sequestering TGF-beta. Decorin also modulates the insulin-like growth factor receptor and the low-density lipoprotein receptor-related protein-1, which indirectly affects the TGF-beta receptor pathway. Gene deletion of decorin causes skin defects, manifested as irregularly shaped collagen type III fibrils of the dermis. Mutations in the decorin gene cause congenital stromal corneal dystrophy. |
| Host | Mouse |
| Species specificity | HU BOV |
| Figure 1 | 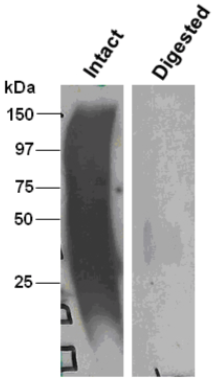 |
| Immunoblot analysis of intact (left) and chondroitinase ABC-digested (right) bovine decorin after SDS-PAGE on 8% gels. | |
| Figure 2 | 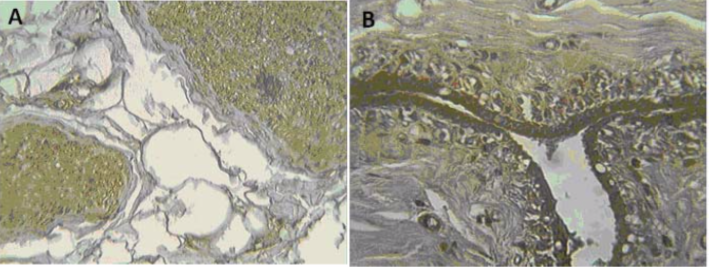 |
| Immunohistochemical analysis of (A) human prostate and (B) human breast. | |
| Product name | Anti Fibromodulin (FM) mAb (Clone 636B12) |
| Cat No | CAC-PRPG-FBM-M01 |
| Description | Fibromodulin, encoded by the FMOD gene, is a member of a family of small interstitial proteoglycans containing a central region composed of leucine-rich repeats with 4 keratan sulfate chains flanked by disulfide-bonded terminal domains. Its core protein is roughly 58 kDa in size and in its fully glycosylated form reaches 150-200 kDa in molecular weight. Fibromodulin has been proposed to participate in the assembly of the extracellular matrix by linking to collagen type I and II and (negatively) controlling their fibrillogenesis in vitro and in vivo (as also confirmed by the altered collagen fibril structure observed in FMOD null mice). Fibromodulin may also influence TGF-beta signaling by sequestering latent TGF-beta in the extracellular matrix. It is recognized to be a primary component of tumor stroma (particularly well documented in epithelial tumors). Recent observations suggest that fibromodulin is a primary prognostic indicator in chronic lymphocytic B-cell leukemia. |
| Host | Mouse |
| Species specificity | HU |
| Figure 1 | 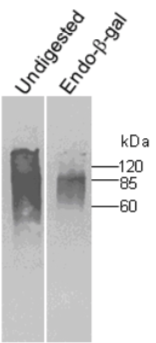 |
| Immunoblot analysis of undigested and endo-betagalactosidase digested human cartilage fibromodulin resolved by SDS-PAGE on 7% gels under reducing conditions (band at 60 kDa presumably corresponds to unglycosylated fibromodulin). | |
| Figure 2 | 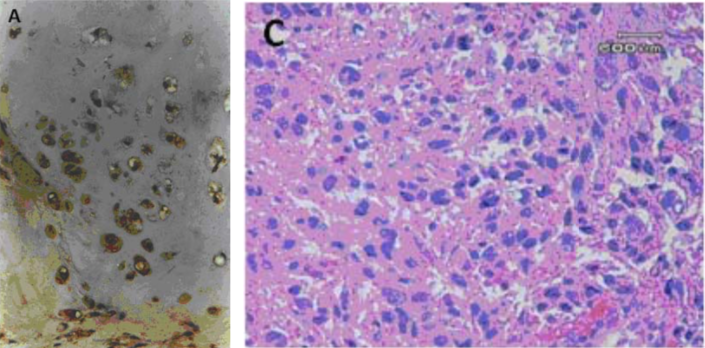 |
| Immunohistochemical analysis. (A) Immunohistochemical staining of human articular cartilage. (C) Immunohistochemical staining of a leiomyosarcoma lesion (labelling is primarily concentrated in the stromal compartment). |
|
| Product name | Anti Neurocan Core Protein (Chondroitin Sulfate Proteoglycan 3) pAb (Rabbit, Antiserum) |
| Cat No | CAC-NU-07-005 |
| Description | Neurocan is a nervous tissue-unique, secretory proteoglycan that carries predominantly chondroitin sulfate side chains. Its expression gradually decreases with the nervous tissue development. In the immature brain, neurocan exists in a full-length form with a 240 kDa-core glycoprotein, whereas it exists totally in proteolytic fragments of the NH2-terminal half (neurocan-N) with a 130 kDa-core glycopeptide and the COOH-terminal half (neurocan-C) with a 150 kDa-core glycopeptide. Neurocan is implicated in the neural network formation, and is a susceptibility factor for bipolar disorder. This proteoglycan is upregulated in the lesion site of the central nervous system, and is a major component of the glial scar. In addition, neurocan-N, not neurocan-C, is detectable in the perineuronal nets of some neurons. This antibody recognizes effectively neurocan-N as well as the full-length neurocan. References: 1) Oohira, A., Matsui, F., Tokita, Y., Yamauchi, S., & Aono, S., Molecular interactions of neural chondroitin sulfate proteoglycans in the brain development, (2000) Arch.Biochem.Biophys., 374, 24-34. 2) Fumiko Matsui, Masako Nishizuka, Yoko Yasuda, Sachiko Aono, Eiji Watanabe, Atsuhiko Oohira. Occurrence of a N-terminal proteolytic fragment of neurocan, not a C-terminal half, in a perineuronal net in the adult rat cerebrum, (1998) Brain Res., 790, 45-51 3) Matsui, F., Watanabe, E., & Oohira, A., Immunological identification of two proteglycan fragments derived from neurocan, a brain-specific chondroitin sulfate proteoglycan, (1994) Neurochem. Int., 25, 425-431. |
| Host | Rabbit |
| Species specificity | MS RT |
| Figure 1 |  |
| Immunoblot analysis of Neurocan peptides pAb and Neurocan peptides mAb (clone 1G2) with samples obtained from 10-day-old rat brain. The PBS-soluble brain CSPG mixture of 10-day-old rats (lane 1) and the brain PBS-extract digested with chondroitinase ABC (lanes 2 and 3) were separated by SDS-PAGE. Neurocan peptides pAb recognized a very diffuse band with the same mobility as that recognized by Neurocan peptides mAb (clone 1G2). Monoclonal antibody (clone 1G2) recognized both 220 and 150 kDa core glycoproteins in the chondroitinase-digested sample. Polyclonal antibody recognized not only the 220 kDa core glycoprotein but also that with a molecular weight of 130 kDa in both (Ref.3). |
|
| Figure 2 | 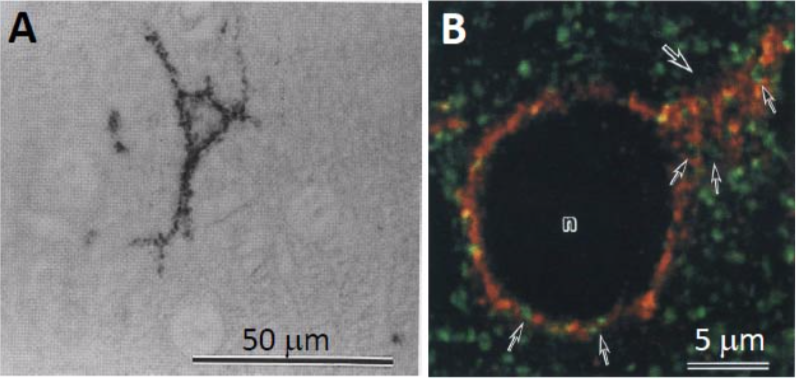 |
| Immunostaining analysis of neurocan distribution in the adult rat cerebral cortex. A. Distribution of neurocan in the adult rat cerebral cortex The cell surfaces of some neurons were immunoreactive for anti-neurocan polyclonal antibody B. Confocal image of the cerebral cortex of an adult rat double-labeled for neurocan-130 (red) and synaptophysin (green). Note neurocan-130 immunoreactivity surrounding a neuronal cell body (n) and a proximal dendrite (large arrow). Neurocan-130 is absent in the axon terminals recognized by anti-synaptophysin (small arrows) (Ref.2). |
|
| Product name | Anti Neurocan Core Protein (Chondroitin Sulfate Proteoglycan 3) mAb (Clone 1G2) |
| Cat No | CAC-NU-07-002 |
| Description |
Neurocan is a nervous tissue-unique, secretory proteoglycan that carries predominantly chondroitin sulfate side chains. Its expression gradually decreases with nervous tissue development. In the immature brain, neurocan exists in a full-length form with a 240 kDa-core glycoprotein, whereas in the mature brain it exists as proteolytic fragments of the NH2-terminal half (neurocan-N) with a 130 kDa-core glycopeptide and the COOH-terminal half (neurocan-C) with a 150 kDa-core glycopeptide. Neurocan is implicated in neural network formation and is a susceptibility factor for bipolar disorder. It is upregulated in central nervous system lesion sites and is a major component of glial scars. This antibody effectively recognizes the COOH-terminal half of rat neurocan core glycoprotein as well as the full length neurocan core glycoprotein. References: |
| Host | MS |
| Species specificity | RT |
| Figure 1 | 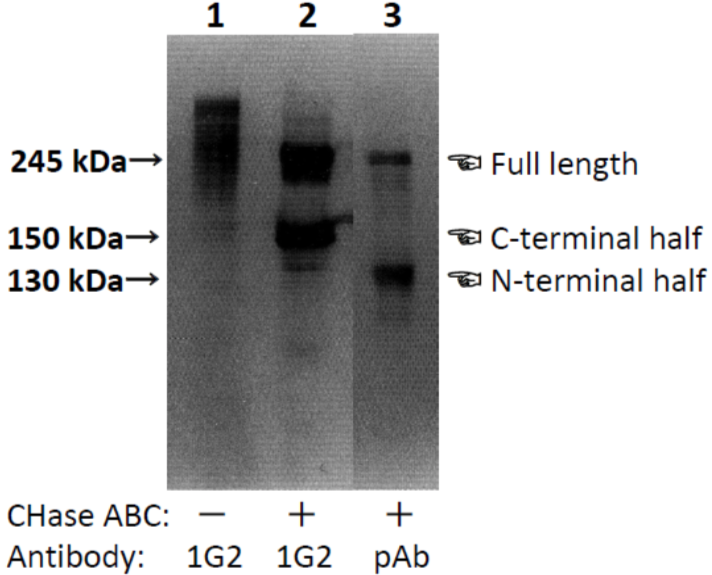 |
| Characterization of neurocan peptide pAb and neurocan mAb (clone 1G2) with samples obtained from 10-day-old rat brain. PBS-soluble brain CSPG mixture of 10-day-old rats (lane 1) and brain PBS-extract digested with chondroitinase ABC (lanes 2 and 3) were separated by SDS-PAGE. Neurocan peptide pAb recognized a very diffuse band with the same mobility as that recognized by neurocan mAb (clone 1G2). Monoclonal antibody (clone 1G2) recognized both 240 and 150 kDa core glycoproteins in the chondroitinase-digested sample. The polyclonal antibody recognized not only the 240 kDa core glycoprotein but also one with a molecular weight of 130 kDa. [from: Neurochem. Int. (1994) 25:425-431.] | |
| Figure 2 | 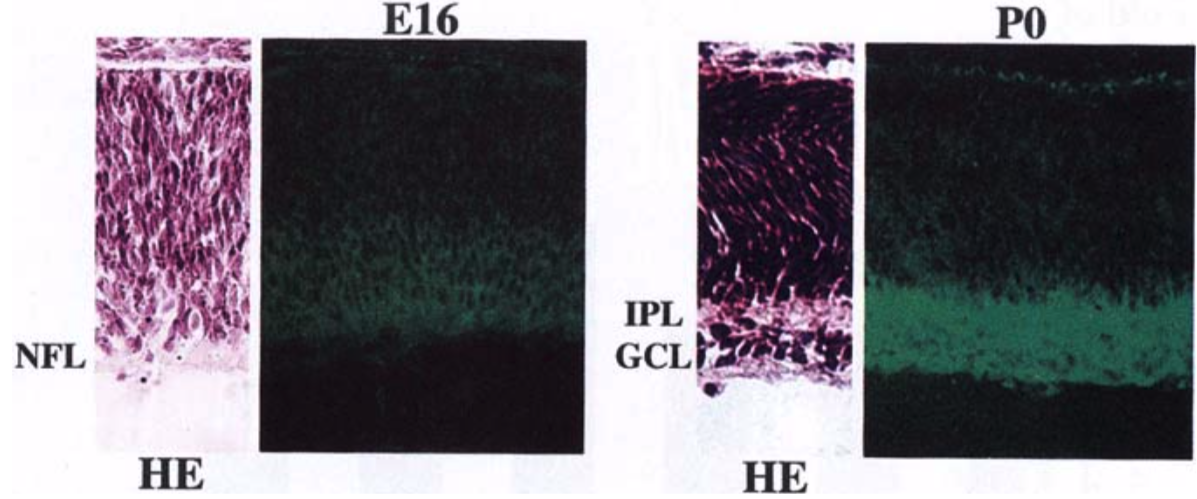 |
| Immunohistochemical staining using monoclonal anti-neurocan antibody (clone 1G2). Cryostat sections of the retina from embryonic day 16 (E16) and newborn (P0) rats were stained with the monoclonal anti-neurocan antibody. Confocal images are shown together with sections stained with hematoxylin-eosin (HE). NFL, nerve fiber layer; IPL, inner plexiform layer; GCL, ganglion cell layer. [from: Invest. Ophthalmol. Vis. Sci. (1999) 40:2350-2359. | |
| Product name | Anti Chondroitin Sulfate Proteoglycan 5 (Neuroglycan C) mAb (Clone C1) |
| Cat No | CAC-NU-07-003 |
| Description | Neuroglycan C (NGC/CALEB) is a transmembrane-type chondroitin sulfate proteoglycan with a core glycoprotein of 120 kDa. It is exclusively expressed and developmentally regulated in the central nervous system. It is altered by addiction to psychostimulants as well as by nerve lesions. NGC is a novel part-time proteoglycan that changes its structure from a proteoglycan form to a non-proteoglycan form without chondroitin sulfate side chains during the development of the cerebellum and retina. The NGC gene is a potential susceptibility factor for schizophrenia. This antibody effectively recognizes the NGC core glycoprotein. References: 1) Watanabe, E. et al. (1995) J. Biol. Chem. 270:26876-26882. 2) Shuo, T. et al. (2004) Glycoconj. J. 20:257-278. 3) Oohira, A. et al. (2004) Glycoconj. J. 21:53-57. 4) Ichihara-Tanaka, K. et al. (2006) J. Biol. Chem. 281:30857-30864. |
| Host | MS |
| Species specificity | RT Other Animals |
| Figure 1 | 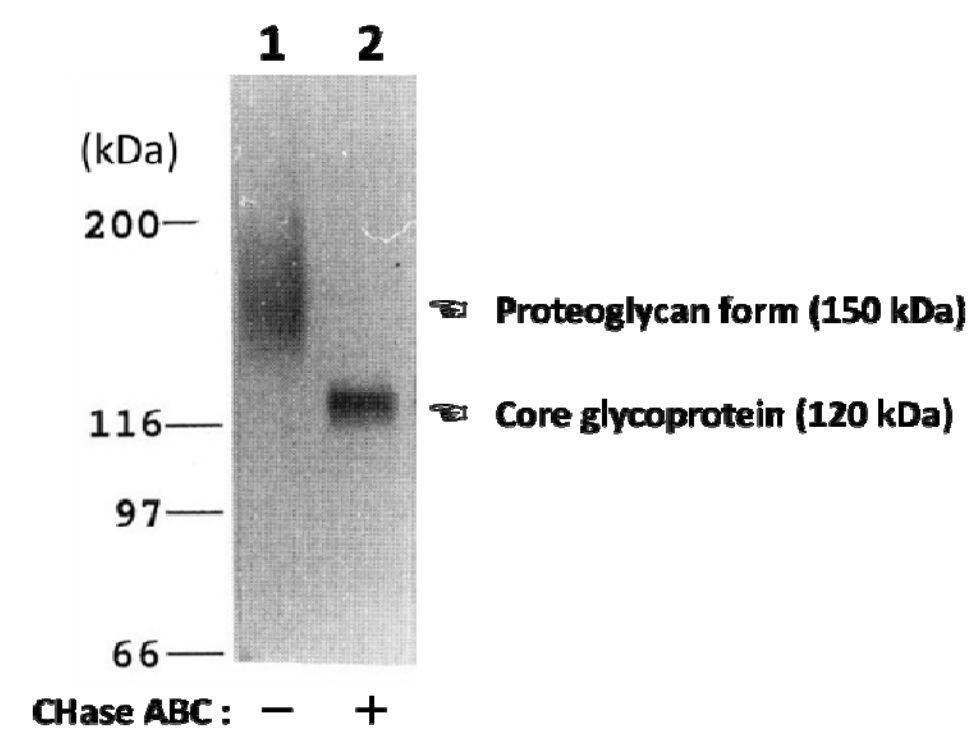 |
| Characterization of the monoclonal anti-neuroglycan C antibody (clone C1). Immunoblot analysis of anti-neuroglycan C antibody on partially purified membrane-bound proteoglycans from brains of 10-day-old rats before (lane 1) and after digestion with chondroitinase ABC (CHase ABC; lane 2). [from: J. Biol. Chem. (1995) 270:26876-26882.] | |
| Product name | Anti Chondroitin Sulfate Proteoglycan 4 (NG2) mAb (Clone 2161D7) |
| Cat No | CAC-PRPG-NG-M01 |
| Description | Chondroitin sulfate proteoglycan 4, also known as melanoma-associated chondroitin sulfate proteoglycan (MCSP) or neuron-glial antigen 2 (NG2), is a chondroitin sulfate proteoglycan that in humans is encoded by the CSPG4 gene. CSPG4 plays a role in stabilizing cell-substratum interactions during early events of melanoma cell spreading on endothelial basement membranes. It represents an integral membrane chondroitin sulfate proteoglycan expressed by human malignant melanoma cells. CSPG4/NG2 is also a hallmark protein of oligodendrocyte progenitor cells (OPCs) and OPC dysfunction has been implicated as a candidate pathophysiological mechanism of familial schizophrenia. A research group investigating the role of genetics in schizophrenia, reported, two rare missense mutations in CSPG4 gene, segregating within families (CSPG4A131T and CSPG4V901G mutations). The researchers also demonstrated that induced pluripotent stem cells(iPSCs)-derived OPCs from CSPG4A131T mutation carriers exhibited abnormal post-translational processing, subcellular localization of the mutant NG2 protein, aberrant cellular morphology, and decreased cell viability and myelination potential. In vivo diffusion tensor imaging of the brain of CSPG4A131T mutation carriers demonstrated reduced white matter integrity compared to unaffected siblings and matched general population controls. |
| Host | Mouse |
| Species specificity | HU |
| Figure 1 | 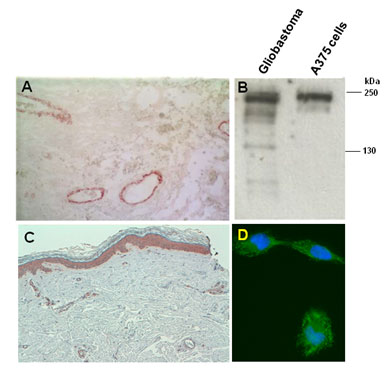 |
| Immunostaining of malignant glioblastoma (A) and cutaneous melanoma (C) lesions. (B) Western blot of whole tissue/cell lysates of a human glioblastoma lesion and the human melanoma A375 cell line after SDS-PAGE under reducing conditions on 4-10% gradient gels. (D) Immunostaining of human melanoma MeWo cells. Nuclear counterstaining was performed with Hoescht. |
|
| Product name | Anti Syndecan-3 (N-Syndecan/Neuroglycan) pAb (Rabbit, Antiserum) |
| Cat No | CAC-NU-07-004 |
| Description | Syndecan is the family name of transmembrane proteoglycans that carry predominantly heparan sulfate side chains. This proteoglycan family consists of four members. N-syndecan (syndecan-3) is the principal member expressed during early postnatal development in both central and peripheral nervous systems. N-syndecan binds various heparin-binding growth factors such as FGFs via the heparan sulfate moiety, and communicates with the cytoskeleton via the cytoplasmic domain of the core protein. N-syndecan plays a pivotal role in formation of the neural network through these molecular interactions. This antibody recognizes effectively the core protein of N-syndecan. References: 1) Watanabe, E., Matsui, F., Keino, H., Ono, K., Kushima, Y., Noda, H.. & Oohira, A., A membrane-bound heparan sulfate proteoglycan that is transiently expressed on growing axons in the rat brain, (1996) J. Neurosci. Res., 44, 84-96. 2) Toba, Y., Horie, M., Sango, K., Takashiki, A., Matsui, F., Oohira, A., & Kawano, H., Expression and immunohistochemical localization of heparan sulfate proteoglycan N-syndecan in the migratory pathway from the rat olfactory placode, (2002) Eur. J. Neurosci., 15, 1-13. |
| Host | Rabbit |
| Species specificity | MS RT |
| Figure 1 |  |
| Immunoblot analysis of the polyclonal anti-N-syndecan antiserum. Western blot using anti-N-syndecan of a partially purified preparation of membrane-bound proteoglycan (lane 1), and that digested with chondroitinase ABC (lane 2), and heparitinase I (lane 3). After the heparitinase I treatment , the immunoreactive band is sifted to an apparent molecular weight of approximately 140 kDa, identical to that of the core glycoprotein of rat N-syndecan. |
|
| Figure 2 | 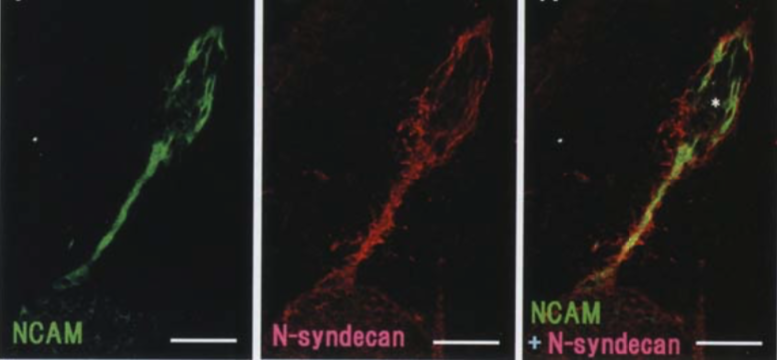 |
| Immunostaining analysis of N-syndecan localization in the developing rat (day 16) vomeronasal system. Double immunofluorescence labelling with anti-NCAM (green) and anti-N-syndecan (red). N-syndecan immunoreactivity surrounds NCAM-immunoreactive vomeronasal axons and their associated cell clusters (asterisk) (Ref.2). |
|
| Product name | Anti Versican Core Protein (Chondroitin Sulfate Proteoglycan 2) mAb (Clone 5C12) |
| Cat No | CAC-PRPG-VS-M01 |
| Description | Versican (also known as PG-M), encoded by the VCAN/CSPG2 gene, is a large extracellular matrix chondroitin sulfate proteoglycan ubiquitously expressed in interstitial matrices of the human body, including brain ECM. It was first described in the bovine aorta by the research groups of Dick Heinegard and Anders Malmstrom’s groups (1982) and shortly after isolated from the chick embryo by Koji Kimata’s group. Cloning of the human VCAN/CSPG2 gene was accomplished in 1989 by Zimmermann and Ruoslahti, who also named the protein as versican in recognition of its versatile modular structure. Versican belongs to the lectican proteoglycan subgroup, to which aggrecan, brevican and neurocan also belong and share the N-terminal (G1) globular domain. This consists of Ig-like loops and two link modules and is responsible for the binding to hyaluronan, which may or may not be further stabilized by link proteins. At least 4 different alternatively spliced versican isoforms are known in higher vertebrates (denoted V0, V1, V2 and V3) while lower vertebrates may have additional ones in part by duplication of the gene. These isoforms are generated through differential utilization of the central core protein regions denoted GAG-α and GAG-β and encompass glycosaminoglycan (chondroitin sulfate) attachment sites. The V0 isoform is the parental one containing both the above “GAG-attachment” exons; the V1 isoform has only the GAG-β domain; the V2 isoform has only the GAG-α domain; and the V3 isoform is void of any GAG attachment domains, and is therefore a GAG-free proteoglycan. This implies that the versican isoform core proteins have a molecular mass range of 50-550 kDa and, when taking also into consideration the extensive glycosylation of the versican core protein, the molecular weights of the different isoforms vary from about 60 kDa to 1,500-2,000 kDa. The C-terminal (G3) globular domain consists of one or two EGF repeats, a C-type lectin module and complement regulatory protein (CRP)-like domain. The C-terminal domain binds a variety of ligands in the ECM and thereby contributes to the macromolecular organization of versican. The role of versican in ECM assembly of elastic matrices, cell adhesion, cell migration, and cell proliferation has been extensively described and its essential role during embryonic development is confirmed by early lethality of murine embryos homozygous for CSPG2 gene deletion. Like other large proteoglycans, versican is processed by multiple MMPs and ADAMTSs and its matrix deposition may be strongly down- or up-regulated in degenerative diseases and cancer. In some tumors its expression pattern has been proposed to have a prognostic value. References: 1) Mazzucato, M., et al., 2002. Vascular PG-M/versican variants promote platelet adhesion at low shear rates and cooperate with collagens to induce aggregation. FASEB J. 16, 1903-1916. 2) Cattaruzza S, et al., 2002. Distribution of PG-M/versican variants in human tissues and de novo expression of isoform V3 upon endothelial cell activation and neoangiogenesis. J.Biol.Chem.277, 47626-47635. 3) Cattaruzza S, Perris R. 2005. Proteoglycan control of cell movement during wound healing and cancer. Matrix Biol. 24, 400-417. |
| Host | Mouse |
| Species specificity | HU BOV |
| Figure 1 | 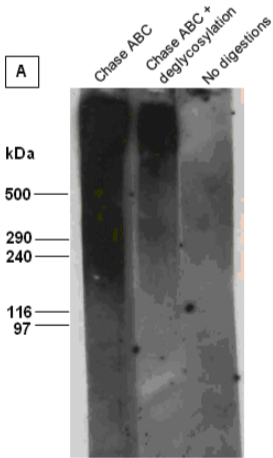 |
| Immunoblot analysis of intact versican (mixture of V1 and V2 isoforms) in its untreated and chondroitinase ABC (Chase ABC)-digested form, or after combined digestion with chondroitinase ABC and a number of exo- and endoglycosydases. The proteoglycan was resolved by SDS-PAGE under reducing conditions on 3-8% linear gradient gels (MW, HiMark Unstained Protein Standard). | |
| Figure 2 | 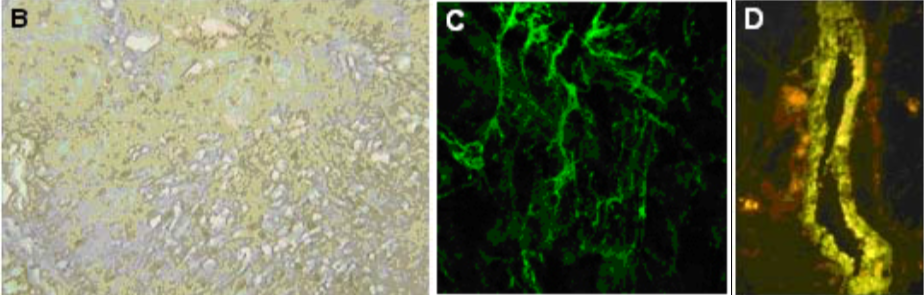 |
| (B) Immunohistochemistry of human normal urinary bladder. (C) Immunostaining of versican in matrix deposited in vitro by human microvascular endothelial cells after TNF stimulation. (D) Immunostaining of versican lining the wall of a large vein in human kidney (PFA-fixed frozen section). |
|
| Product name | Anti Versican Core Protein (Chondroitin Sulfate Proteoglycan 2) mAb (Clone 4C5) |
| Cat No | CAC-PRPG-VS-M02 |
| Description | Versican (also known as PG-M), encoded by the VCAN/CSPG2 gene, is a large extracellular matrix chondroitin sulfate proteoglycan ubiquitously expressed in interstitial matrices of the human body, including brain ECM. It was first described in the bovine aorta by the research groups of Dick Heinegard and Anders Malmstrom’s groups (1982) and shortly after isolated from the chick embryo by Koji Kimata’s group. Cloning of the human VCAN/CSPG2 gene was accomplished in 1989 by Zimmermann and Ruoslahti, who also named the protein as versican in recognition of its versatile modular structure. Versican belongs to the lectican proteoglycan subgroup, to which aggrecan, brevican and neurocan also belong and share the N-terminal (G1) globular domain. This consists of Ig-like loops and two link modules and is responsible for the binding to hyaluronan, which may or may not be further stabilized by link proteins. At least 4 different alternatively spliced versican isoforms are known in higher vertebrates (denoted V0, V1, V2 and V3) while lower vertebrates may have additional ones in part by duplication of the gene. These isoforms are generated through differential utilization of the central core protein regions denoted GAG-α and GAG-β and encompass glycosaminoglycan (chondroitin sulfate) attachment sites. The V0 isoform is the parental one containing both the above “GAG-attachment” exons; the V1 isoform has only the GAG-β domain; the V2 isoform has only the GAG-α domain; and the V3 isoform is void of any GAG attachment domains, and is therefore a GAG-free proteoglycan. This implies that the versican isoform core proteins have a molecular mass range of 50-550 kDa and, when taking also into consideration the extensive glycosylation of the versican core protein, the molecular weights of the different isoforms vary from about 60 kDa to 1,500-2,000 kDa. The C-terminal (G3) globular domain consists of one or two EGF repeats, a C-type lectin module and complement regulatory protein (CRP)-like domain. The C-terminal domain binds a variety of ligands in the ECM and thereby contributes to the macromolecular organization of versican. The role of versican in ECM assembly of elastic matrices, cell adhesion, cell migration, and cell proliferation has been extensively described and its essential role during embryonic development is confirmed by early lethality of murine embryos homozygous for CSPG2 gene deletion. Like other large proteoglycans, versican is processed by multiple MMPs and ADAMTSs and its matrix deposition may be strongly down- or up-regulated in degenerative diseases and cancer. In some tumors its expression pattern has been proposed to have a prognostic value. References: 1) Mazzucato, M., et al., 2002. Vascular PG-M/versican variants promote platelet adhesion at low shear rates and cooperate with collagens to induce aggregation. FASEB J. 16, 1903-1916. 2) Cattaruzza S, et al., 2002. Distribution of PG-M/versican variants in human tissues and de novo expression of isoform V3 upon endothelial cell activation and neoangiogenesis. J.Biol.Chem.277, 47626-47635. 3) Cattaruzza S, Perris R. 2005. Proteoglycan control of cell movement during wound healing and cancer. Matrix Biol. 24, 400-417. |
| Host | Mouse |
| Species specificity | HU BOV |
| Figure 1 |  |
| Immunoblot analysis of intact versican (mixture of V1 and V2 isoforms) prior to (Intact) and after combined chondroitinase ABC (Chase ABC) and endo-galactosidase digestion (Digested). The proteoglycan was resolved by SDS-PAGE under reducing conditions on 3-8% linear gradient gels (MW, HiMark Unstained Protein Standard). Banding pattern depends upon the isoforms and is often complex. In the intact forms, i.e. without removal of GAGs, isoforms V0, V1 and V2 do not enter conventional polyacrylamide gels and therefore alternative gel types are strongly recommended. Following chondroitinase-digestion and extensive enzymatic deglycosylation, most isoforms still show complex, smeared banding patterns. |
|
| Figure 2 | 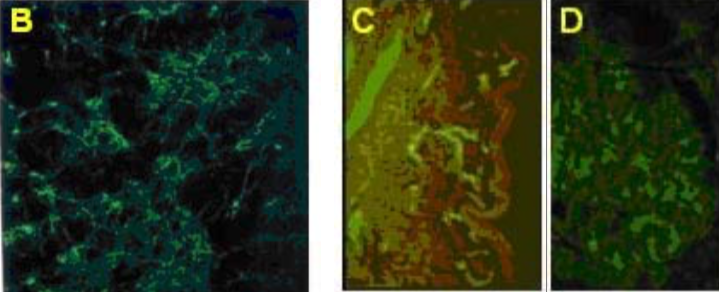 |
| (B) Immunocytochemistry on cultured smooth muscle cells showing versican distribution in the ECM deposited by the cells. (C) Immunostaining of versican in normal human skin. (D) Immunostaining of versican distribution in the Bowman capsule of a normal human kidney (PFA-fixed frozen section). |
|
| Product name | Anti Versican Core Protein (Chondroitin Sulfate Proteoglycan 2) mAb (Clone 2B3) |
| Cat No | CAC-PRPG-VS-M03 |
| Description | Versican (also known as PG-M), encoded by the VCAN/CSPG2 gene, is a large extracellular matrix chondroitin sulfate proteoglycan ubiquitously expressed in interstitial matrices of the human body, including brain ECM. It was first described in the bovine aorta by the research groups of Dick Heinegard and Anders Malmstrom’s groups (1982) and shortly after isolated from the chick embryo by Koji Kimata’s group. Cloning of the human VCAN/CSPG2 gene was accomplished in 1989 by Zimmermann and Ruoslahti, who also named the protein as versican in recognition of its versatile modular structure. Versican belongs to the lectican proteoglycan subgroup, to which aggrecan, brevican and neurocan also belong and share the N-terminal (G1) globular domain. This consists of Ig-like loops and two link modules and is responsible for the binding to hyaluronan, which may or may not be further stabilized by link proteins. At least 4 different alternatively spliced versican isoforms are known in higher vertebrates (denoted V0, V1, V2 and V3) while lower vertebrates may have additional ones in part by duplication of the gene. These isoforms are generated through differential utilization of the central core protein regions denoted GAG-α and GAG-β and encompass glycosaminoglycan (chondroitin sulfate) attachment sites. The V0 isoform is the parental one containing both the above “GAG-attachment” exons; the V1 isoform has only the GAG-β domain; the V2 isoform has only the GAG-α domain; and the V3 isoform is void of any GAG attachment domains, and is therefore a GAG-free proteoglycan. This implies that the versican isoform core proteins have a molecular mass range of 50-550 kDa and, when taking also into consideration the extensive glycosylation of the versican core protein, the molecular weights of the different isoforms vary from about 60 kDa to 1,500-2,000 kDa. The C-terminal (G3) globular domain consists of one or two EGF repeats, a C-type lectin module and complement regulatory protein (CRP)-like domain. The C-terminal domain binds a variety of ligands in the ECM and thereby contributes to the macromolecular organization of versican. The role of versican in ECM assembly of elastic matrices, cell adhesion, cell migration, and cell proliferation has been extensively described and its essential role during embryonic development is confirmed by early lethality of murine embryos homozygous for CSPG2 gene deletion. Like other large proteoglycans, versican is processed by multiple MMPs and ADAMTSs and its matrix deposition may be strongly down- or up-regulated in degenerative diseases and cancer. In some tumors its expression pattern has been proposed to have a prognostic value. References: 1) Wight TN., Curr Opin Cell Biol.2002 Oct;14(5):617-23. PMID:12231358 2) Wu YJ, et all., Cell Res. 2005 Jul;15(7):483-94. PMID:16045811. 3) Cattaruzza S, et all., J Biol Chem. 2002 Dec 6;277(49):47626-35..PMID:12221092 4) Garusi E, et all., Cell Mol Life Sci. 2012 Feb;69(4):553-79. PMID:21964924 5) Heinegard D, et all., Biochem J. 1985 Aug 15;230(1):181-94. PMID:4052035 6) Kimata K, et all., J Biol Chem.1986 Oct 15;261(29):13517-25. PMID:3759975 7) Morgelin M., et all., J Biol Chem.1989 Jul 15;264(20):12080-90. PMID:2745430 8) Zimmermann DR, et all., EMBO J. 1989 Oct;8(10):2975-81. PMID:2583089 |
| Host | Mouse |
| Species specificity | HU BOV |
| Figure 1 | 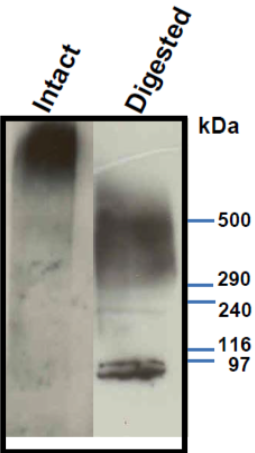 |
| Immunoblot analysis of purified human aorta versican resolved by SDS-PAGE on 3-8% linear gradient gels (MW, HiMark Unstained Protein Standard) before (Intact) and after combined chondroitinase ABC and endo-b-galactosidase pretreatment (Digested). * Banding pattern observed by immunoblotting depends upon the isoforms and is often complex. In the intact forms, i.e. without removal of GAGs, V0, V1 and V2 do not enter acrylamide gels and therefore agarose gels are recommended. Following chondroitinase-digestion and extensive enzymatic deglycosylation, most isoforms still show complex, smeared banding patterns. |
|
| Figure 2 | 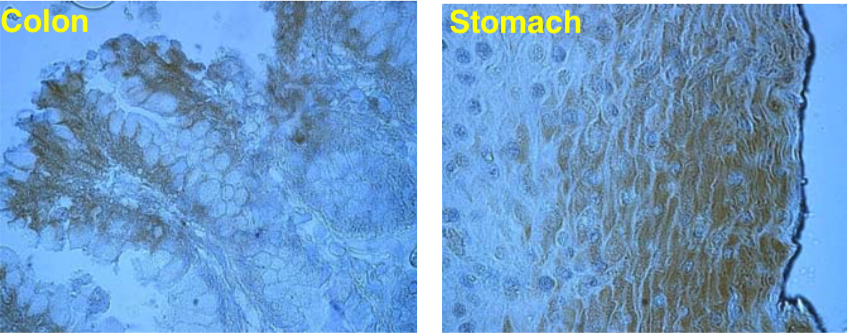 |
| Immunolocalization of versican isoforms (primarily a mixture of V1 and V2 isoforms) recognized by mAb 2B3 in the indicated human adult tissues (peroxidase reaction). * mAb 2B3 detects specific versican V0-V2 isoforms widely distributed in connective tissues and particularly concentrated in vascular structures. Chondroitinase ABC pre-digestion of the sections may affect the staining pattern. |
|
| Product name | Anti Versican Core Protein (Chondroitin Sulfate Proteoglycan 2) mAb (Clone 6B10) |
| Cat No | CAC-PRPG-VS-M04 |
| Description | Versican (also known as PG-M), encoded by the VCAN/CSPG2 gene, is a large extracellular matrix chondroitin sulfate proteoglycan ubiquitously expressed in interstitial matrices of the human body, including brain ECM. It was first described in the bovine aorta by the research groups of Dick Heinegard and Anders Malmstrom’s groups (1982) and shortly after isolated from the chick embryo by Koji Kimata’s group. Cloning of the human VCAN/CSPG2 gene was accomplished in 1989 by Zimmermann and Ruoslahti, who also named the protein as versican in recognition of its versatile modular structure. Versican belongs to the lectican proteoglycan subgroup, to which aggrecan, brevican and neurocan also belong and share the N-terminal (G1) globular domain. This consists of Ig-like loops and two link modules and is responsible for the binding to hyaluronan, which may or may not be further stabilized by link proteins. At least 4 different alternatively spliced versican isoforms are known in higher vertebrates (denoted V0, V1, V2 and V3) while lower vertebrates may have additional ones in part by duplication of the gene. These isoforms are generated through differential utilization of the central core protein regions denoted GAG-α and GAG-β and encompass glycosaminoglycan (chondroitin sulfate) attachment sites. The V0 isoform is the parental one containing both the above “GAG-attachment” exons; the V1 isoform has only the GAG-β domain; the V2 isoform has only the GAG-α domain; and the V3 isoform is void of any GAG attachment domains, and is therefore a GAG-free proteoglycan. This implies that the versican isoform core proteins have a molecular mass range of 50-550 kDa and, when taking also into consideration the extensive glycosylation of the versican core protein, the molecular weights of the different isoforms vary from about 60 kDa to 1,500-2,000 kDa. The C-terminal (G3) globular domain consists of one or two EGF repeats, a C-type lectin module and complement regulatory protein (CRP)-like domain. The C-terminal domain binds a variety of ligands in the ECM and thereby contributes to the macromolecular organization of versican. The role of versican in ECM assembly of elastic matrices, cell adhesion, cell migration, and cell proliferation has been extensively described and its essential role during embryonic development is confirmed by early lethality of murine embryos homozygous for CSPG2 gene deletion. Like other large proteoglycans, versican is processed by multiple MMPs and ADAMTSs and its matrix deposition may be strongly down- or up-regulated in degenerative diseases and cancer. In some tumors its expression pattern has been proposed to have a prognostic value. References: 1) Wight TN., Curr Opin Cell Biol.2002 Oct;14(5):617-23. PMID:12231358 2) Wu YJ, et all., Cell Res. 2005 Jul;15(7):483-94. PMID:16045811. 3) Cattaruzza S, et all., J Biol Chem. 2002 Dec 6;277(49):47626-35..PMID:12221092 4) Garusi E, et all., Cell Mol Life Sci. 2012 Feb;69(4):553-79. PMID:21964924 5) Heinegard D, et all., Biochem J. 1985 Aug 15;230(1):181-94. PMID:4052035 6) Kimata K, et all., J Biol Chem.1986 Oct 15;261(29):13517-25. PMID:3759975 7) Morgelin M., et all., J Biol Chem.1989 Jul 15;264(20):12080-90. PMID:2745430 8) Zimmermann DR, et all., EMBO J. 1989 Oct;8(10):2975-81. PMID:2583089 |
| Host | Mouse |
| Species specificity | HU BOV |
| Figure 1 | 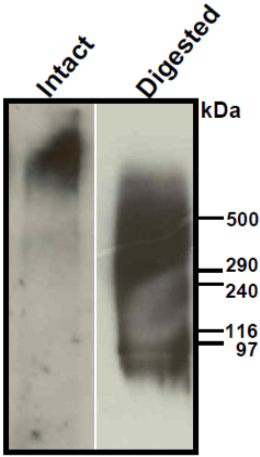 |
| Immunoblot analysis of purified human aorta versican (primarily a mixture of V1 and V2 isoforms) resolved by SDS-PAGE on 3-8% linear gradient gels under reducing conditions (MW, HiMark Unstained Protein Standard) before (Intact) and after combined chondroitinase ABC and endo-b-galactosidase pretreatment (Digested). * Banding pattern observed by immunoblotting depends upon the isoforms and is often complex. In the intact forms, i.e. without removal of GAGs, V0, V1 and V2 do not enter acrylamide gels and therefore agarose gels are recommended. Following chondroitinase-digestion and extensive enzymatic deglycosylation, most isoforms still show complex, smeared banding patterns. |
|
| Figure 2 | 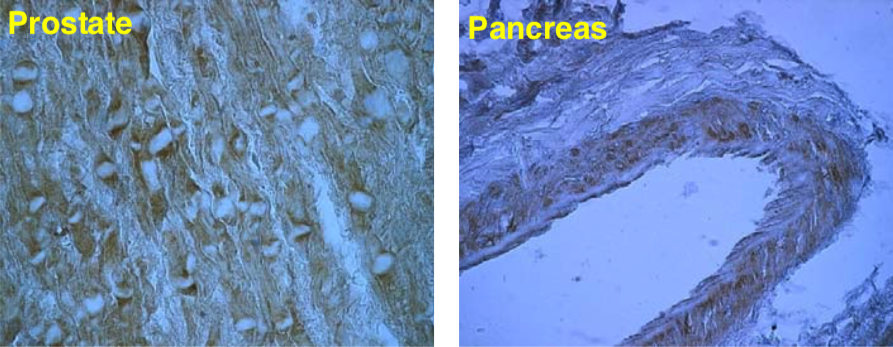 |
| Immunolocalization of versican isoforms recognized by mAb 6B10 in the indicated human adult tissues (peroxidase reaction). * mAb 6B10 detects specific versican V0-V2 isoforms widely distributed in connective tissues and particularly concentrated in vascular structures. Chondroitinase ABC pre-digestion of the sections may affect the staining pattern. |
|
| Matrix and basement membrane |
|
| As mentioned earlier, basal laminae are flexible, thin (40–120 nm thick) mats of specialized extracellular matrix that underlie all epithelial cell sheets and tubes. They also surround individual muscle cells, fat cells, and Schwann cells (which wrap around peripheral nerve cell axons to form myelin). The basal lamina thus separates these cells and epithelia from the underlying or surrounding connective tissue. In other locations, such as the kidney glomerulus, a basal lamina lies between two cell sheets and functions as a highly selective filter. Basal laminae have more than simple structural and filtering roles, however. They are able to determine cell polarity, influence cell metabolism, organize the proteins in adjacent plasma membranes, promote cell survival, proliferation, or differentiation, and serve as specific highways for cell migration. The basal lamina is synthesized largely by the cells that rest on it. In some multilayered epithelia, such as the stratified squamous epithelium that forms the epidermis of the skin, the basal lamina is tethered to the underlying connective tissue by specialized anchoring fibrils made of type VII collagen molecules. The term basement membrane is often used to describe the composite of the basal lamina and this layer of collagen fibrils. In one type of skin disease, these connections are either absent or destroyed, and the epidermis and its basal lamina become detached from the underlying connective tissue, causing blistering. Although its precise composition varies from tissue to tissue and even from region to region in the same lamina, most mature basal laminae contain type IV collagen, the large heparan sulfate proteoglycan perlecan, and the glycoproteins laminin and nidogen (also called entactin). Type IV collagens exist in several isoforms. They all have a more flexible structure than the fibrillar collagens; their triple-stranded helix is interrupted in 26 regions, allowing multiple bends. They are not cleaved after secretion but interact via their uncleaved terminal domains to assemble extracellularly into a flexible, sheet-like, multilayered network. Early in development, basal laminae contain little or no type IV collagen and consist mainly of laminin molecules. Laminin-1 (classical laminin) is a large, flexible protein composed of three very long polypeptide chains (α, β, and γ) arranged in the shape of an asymmetric cross and held together by disulfide bonds. Several isoforms of each type of chain can associate in different combinations to form a large family of laminins. The laminin γ-1 chain is a component of most laminin heterotrimers, and mice lacking it die during embryogenesis because they are unable to make a basal lamina. Like many other proteins in the extracellular matrix, the laminin in basement membranes consists of several functional domains: one binds to perlecan, one to nidogen, and two or more to laminin receptor proteins on the surface of cells. Like type IV collagen, laminins can self-assemble in vitro into a felt-like sheet, largely through interactions between the ends of the laminin arms. As nidogen and perlecan can bind to both laminin and type IV collagen, it is thought that they connect the type IV collagen and laminin networks. In tissues, laminins and type IV collagen preferentially polymerize while bound to receptors on the surface of the cells producing the proteins. Many of the cell-surface receptors for type IV collagen and laminin are members of the integrin family. Another important type of laminin receptor is the transmembrane protein dystroglycan, which, together with integrins, may organize the assembly of the basal lamina. [from: Alberts B, Johnson A, Lewis J, et al. Molecular Biology of the Cell. 4th edition. New York: Garland Science; 2002. The Extracellular Matrix of Animals. Available from: https://www.ncbi.nlm.nih.gov/books/NBK26810/] |
|
| Product name | Anti Laminin/Nidogen complex mAb (Clone 331G3) |
| Cat No | CAC-PRPG-NDG-M01 |
| Description | Nidogen-1 (originally named entactin) and its homologue nidogen-2 are ubiquitous and highly conserved components of basement membranes (BMs), though nidogen-2 shows more restricted expression patterns throughout development and some tissue specificity in the adult. In BMs nidogen play a fundamental role in linking collagen type IV-laminin networks and in bridging these networks with oligomers of perlecan, matrilins and fibulins. Nidogen-1 is encoded by the NID1 gene and is translated as a 158 kDa globular protein containing three distinct globules with diverse ligand binding specificity. Gene deletion of either nidogen does not disrupt developmental BM assembly, whereas ablation of both NID1 and NID2 murine genes leads to BM abnormalities and prenatal death. Alterations in nidogen-1 expression have been associated with neoplastic transformation (e.g. hepatocarcinoma, melanoma, bladder carcinoma, renal carcinoma, gastrointestinal cancer), skin, renal, retinal, muscle and connective tissue degenerative diseases. References: 1) Carlin B, et all., J Biol Chem. 1981 May 25;256(10):5209-14. PMID: 6262321 2) Timpl R, et all., Eur J Biochem. 1983 Dec 15;137(3):455-65. PMID: 6420150 3) Kohfeldt E, et all., J Mol Biol. 1998 Sep 11;282(1):99-109. PMID: 9733643 4) Ho MS, et all., Microsc Res Tech. 2008 May;71(5):387-95. PMID: 18219668 5) Breitkreutz D, et all., Biomed Res Int. 2013;2013:179784. PMID: 23586018 6) Murshed M, et all., Mol Cell Biol. 2000 Sep;20(18):7007-12. PMID: 10958695 7) Miosge N, et all., Matrix Biol. 2002 Nov;21(7):611-21. PMID: 12475645 8) Bader BL, et all., Mol Cell Biol. 2005 Aug;25(15):6846-56. PMID: 16024816 |
| Host | Mouse |
| Species specificity | HU |
| Figure 1 | 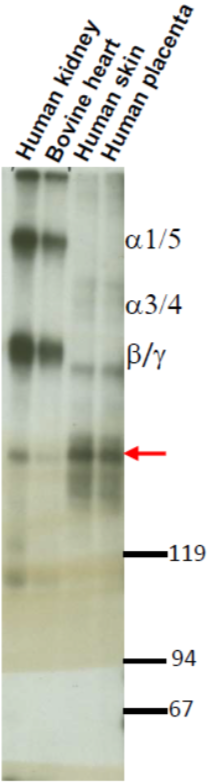 |
| Immunoprecipitation immunoblot analysis of biotinylated laminin-nidogen complexes resolved by SDS-PAGE on 5% gels under reducing conditions and visualized by chemiluminescence; red arrow points to the nidogen band, whereas upper bands correspond to a, b and g laminin chains. | |
| Figure 2 | 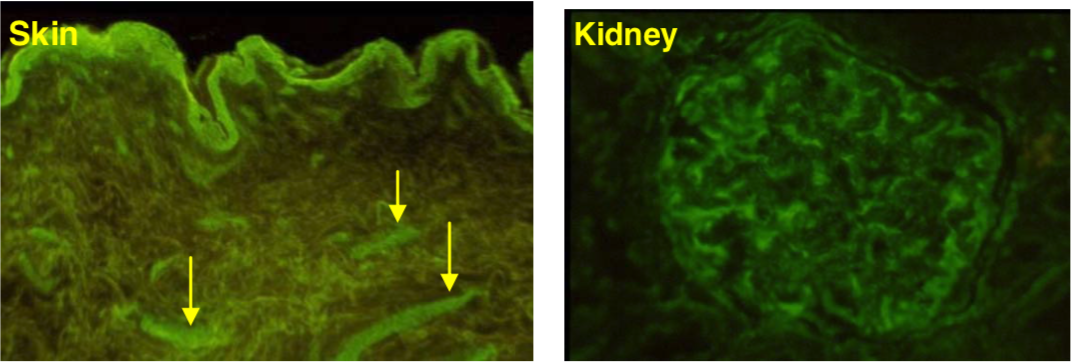 |
| Immunostaining analysis of nidogen localization in basement membranes of human skin and kidney Bowman’s capsule in fresh-frozen sections (arrows point microvascular vessels). * mAb 331G3 stains basement membranes of all major organs/tissues of the human body. |
|
| Product name | Anti Laminin Subunit Alpha-3 mAb (Clone BM515) |
| Cat No | CAC-NU-01-LA3 |
| Description | Laminins, a family of extracellular matrix glycoproteins, are the major noncollagenous constituents of basement membranes. They have been implicated in a wide variety of biological processes including cell adhesion, differentiation, migration, signaling, neurite outgrowth and metastasis. Laminins are heterotrimers composed of 3 non-identical chains that adopt a cruciform structure with 3 short arms, each formed by a different chain, and a long arm composed of all 3 chains. Each laminin chain is a multidomain protein encoded by a distinct gene and in some cases undergoes alternative splicing to give rise to separate chain variants. References: 1) Hirako Y., Yoshino K., Zillikens D., Owaribe K. Extracellular cleavage of bullous pemphigoid antigen 180/type XVII collagen and its involvement in hemidesmosomal disassembly. J Biochem. 2003 Feb;133(2):197-206. PMID: 12761182 2) Uematsu J., Nishizawa Y., Hirako Y., Kitamura K., Usukura J., Miyata T., Owaribe K. Both type-I hemidesmosomes and adherens-type junctions contribute to the cell-substratum adhesion system in myoepithelial cells. Eur J Cell Biol. 2005 Mar;84(2-3):407-15. PMID: 15819417 3) Owaribe K, Nishizawa Y, Franke WW. Isolation and characterization of hemidesmosomes from bovine corneal epithelial cells. Exp Cell Res. 1991 Feb;192(2):622-30. PMID:1988297 |
| Host | Mouse |
| Species specificity | HU BOV RAB |
| Figure 1 | 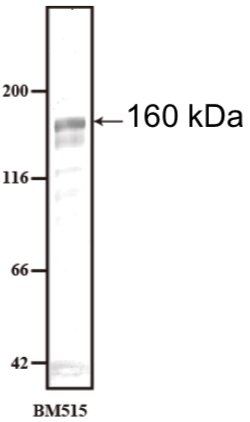 |
| Immunoblot analysis of extracellular matrix made from Human keratinocyte cell line HaCaT using Anti-Laminin alpha 3 (clone BM515). Arrows indicate Laminin alpha 3 (approximately 160kDa). Non-processed form of Laminin α3 chain might be detected around 190kDa depending on the specimen. |
|
| Figure 2 | 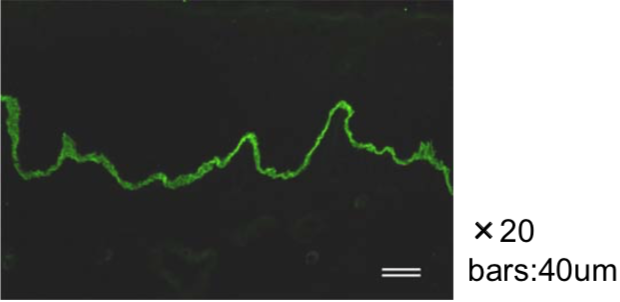 |
| Immunofluorescence microscopy of human skin using Anti-Laminin alpha 3 (clone BM515, 1/200 dilution). Epidermal basement membrane containing Laminin alpha 3 chain is stained green. |
|
| Figure 3 | 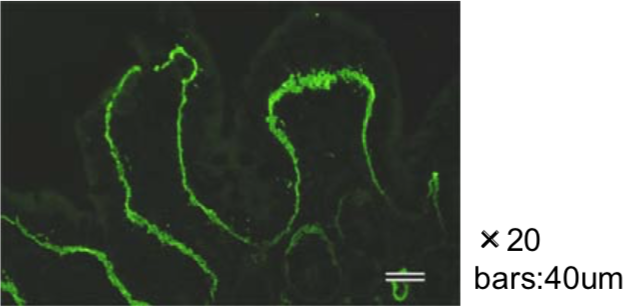 |
| Immunofluorescence microscopy of rabbit large intestine using anti Laminin alpha 3 (clone BM515, 1/200 dilution). Large intestine basement membrane containing Laminin α3 chain is stained green. |
|
| Product name | Anti Laminin Alpha-4 mAb (Clone 652C4) |
| Cat No | CAC-PRPG-LA4-M01 |
| Description | Laminins, a family of extracellular matrix glycoproteins, are the major noncollagenous constituents of basement membranes. They have been implicated in a wide variety of biological processes including cell adhesion, differentiation, migration, signaling, neurite outgrowth and metastasis. Laminins are heterotrimers composed of 3 non-identical chains that adopt a cruciform structure with 3 short arms, each formed by a different chain, and a long arm composed of all 3 chains. Each laminin chain is a multidomain protein encoded by a distinct gene and in some cases undergoes alternative splicing to give rise to separate chain variants. References: 1) Hirako Y., Yoshino K., Zillikens D., Owaribe K. Extracellular cleavage of bullous pemphigoid antigen 180/type XVII collagen and its involvement in hemidesmosomal disassembly. J Biochem. 2003 Feb;133(2):197-206. PMID: 12761182 2) Uematsu J., Nishizawa Y., Hirako Y., Kitamura K., Usukura J., Miyata T., Owaribe K. Both type-I hemidesmosomes and adherens-type junctions contribute to the cell-substratum adhesion system in myoepithelial cells. Eur J Cell Biol. 2005 Mar;84(2-3):407-15. PMID: 15819417 3) Owaribe K, Nishizawa Y, Franke WW. Isolation and characterization of hemidesmosomes from bovine corneal epithelial cells. Exp Cell Res. 1991 Feb;192(2):622-30. PMID:1988297 |
| Host | Mouse |
| Species specificity | HU |
| Product name | Anti Collagen Alpha-1(VII) Chain mAb (Clone BML39) |
| Cat No | CAC-NU-01-CO7 |
| Description | This gene encodes the alpha chain of type VII collagen. The type VII collagen fibril, composed of three identical alpha collagen chains, is restricted to the basement zone beneath stratified squamous epithelia. It functions as an anchoring fibril between the external epithelia and the underlying stroma. Mutations in this gene are associated with all forms of dystrophic epidermolysis bullosa. The inherited disease, dystrophic epidermolysis bullosa, is caused by recessive or dominant mutations in COL7A1. In the absence of mutations, however, an autoimmune response against type VII collagen can result in an acquired form of this disease called epidermolysis bullosa acquisita. Type VII collagen is also found in the retina; its function in this organ is unknown. Collagen, type VII, alpha 1 has been shown to interact with Laminin 5 and Fibronectin. Source: Nagoya University Graduate School of Science Department of Life Science Cell Biology Group Lecturer Mr. Yoshiko Hirako. References: 1) Uematsu J., et al. Eur J Cell Biol., 84: 407-415 (2005). 2) Hirako Y., et al. J. Biol. Chem., 273:9711-9717 (1998). |
| Host | Mouse |
| Species specificity | HU BOV RAB POR |
| Figure 1 | 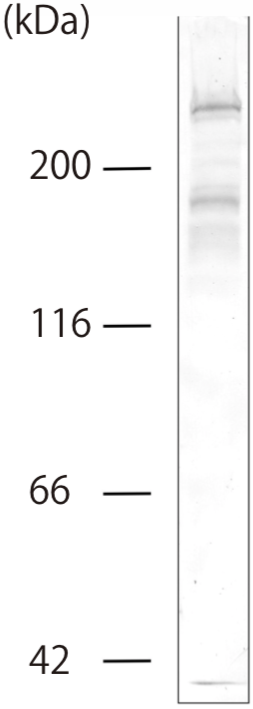 |
| Immunoblot analysis. Concentrated conditioned medium prepared from BMGE+H cells was immunoblotted with BML39 antibody (1:50 dilution). BML39 antibody detected type-7 collagen as a band at approximately 300 kDa (full-length) and degraded fragments around 170 kDa Conditioned medium was concentrated by ammonia sulfate precipitation as described previously (ref. 2). Polypeptides were separated by SDS-PAGE (7.5%). |
|
| Figure 2 | 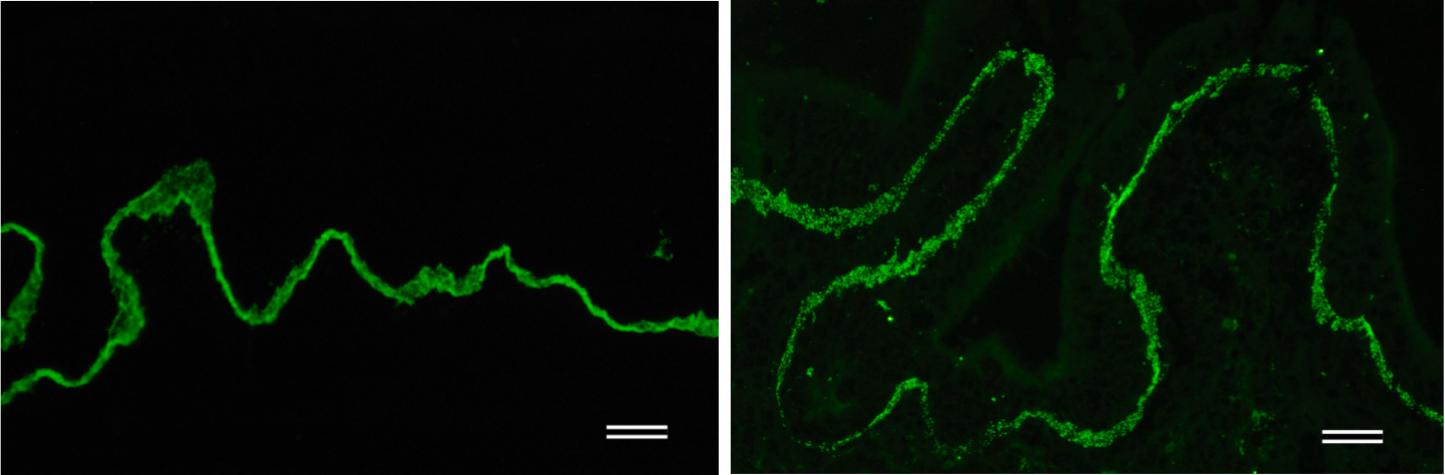 |
| Immunofluorescence microscopy of human skin and rabbit large intestine. Frozen sections of human skin (left) and rabbit large intestine (right) were stained with BML39 antibody at 1:200 dilution. The antibody revealed the location of type VII collagen at basement membranes. Bars = 40 um. Frozen sections were prepared as described previously (ref. 2). |
|
| Product name | Anti Collagen Alpha-1(XII) Chain mAb (Clone 378D5) |
| Cat No | CAC-PRPG-CO12-M01 |
| Description | Type XII collagen is a member of the FACIT (fibril-associated collagens with interrupted triple helices) collagen subfamily currently composed of 9 members (collagen types VII, IX, XII, XIV, XVI, XIX, XX, and XXI). Type XII collagen is a homotrimer composed of three alpha-1(XII) chains with an approximate Mr of 220 kDa. This collagen was originally found in prevalent association with type I collagen in tendons, ligaments, perichondrium, and periosteum and all dense connective tissues, but more recent studies also document association of the collagen with basement membranes. The type XII-type I collagen connection is thought to modify the interactions between collagen type I fibrils and the surrounding matrix such as to create specific macromolecular configuration of the ECM. Alternatively spliced transcript variants encoding different isoforms of the collagen have been identified. Rotary shadowing/TEM analyses shows a structure with a thin 75-nm tail, which is frequently kinked, attached through a central globule to three finger-like projections, each 60 nm long. |
| Host | Mouse |
| Species specificity | HU BOV Avian |
| Product name | Anti Proprotein Convertase Subtilisin/Kexin Type 6 (PACE4) - HomoB domain pAb (Rabbit, Antiserum) |
| Cat No | CAC-SK-T01-001 |
| Description | PACE4, PC6 and furin are potent subtilisin-like proprotein convertases (SPCs) which are responsible for the activation of transforming growth factor-beta (TGF beta)-related factors such as bone morphogenetic proteins. Heparan sulfate proteoglycan within the extracellular matrix (ECM) is known to regulate the biological activity of various differentiation factors including TGF beta-related molecules. PACE4 binds tightly to heparin and its heparin-binding region was found to be a cationic stretch of amino acids between residues 743 and 760. Furthermore, PACE4 was detected in the extracellular material fraction of the HEK293 cells, defined as the material remaining on the culture plate following the removal of the cells from the plate. PACE4 bound to the extracellular fraction was selectively dislodged by heparin into the culture medium. Heparin has no inhibitory activity against PACE4. Similarly, PC6A is also able to bind to heparin, whereas soluble furin does not. In human placenta, PACE4 is mainly present in syncytiotrophoblasts and can be released by heparin. These results suggest that PACE4 and PC6 are unique SPC family proteases that anchor heparan sulfate proteoglycans at the ECM. The interaction between PACE4 and heparan sulfate proteoglycans might play an important role in the delicate spatiotemporal regulation of TGF beta-related factors' biological activity. [from: Tsuji A., Sakurai K., Kiyokage E., Yamazaki T., Koide S., Toida K., Matsuda Y. Secretory proprotein convertases PACE4 and PC6A are heparin-binding proteins which are localized in the extracellular matrix: Potential role of PACE4 in the activation of proproteins in the extracellular matrix (2003) Biochimica et Biophysica Acta - Proteins and Proteomics 1645(1) 95-104.] Source: Professor Akihiko Tsuji, Faculty of Engineering, Tokushima University. References: 1) Nagahama et al. (1998) Biosynthetic processing and quaternary interaction of proprotein convertase SPC4 (PACE4). FEBS Letters 434, 155-159. 2) Tsuji et al. (2003) Secretory proprotein convertases PACE4 and PC6A are heparin-binding proteins which are localized in the extracellular matrix. Biochim. Biophys. Acta 1645, 95-104. |
| Host | Rabbit |
| Species specificity | HU |
| Product name | Anti Proprotein Convertase Subtilisin/Kexin Type 6 (PACE4) - Propeptide pAb (Rabbit, Antiserum) |
| Cat No | CAC-SK-T01-002 |
| Description | PACE4A is a member of the mammalian subtilisin-like proprotein convertase family which is responsible for the proteolytic activation of precursors into their biologically active forms. Previously we reported that the maturation of proPACE4A occurs via a intramolecular autoactivation and cleavage of the propeptide is a rate-limiting step for the secretion of PACE4A (Nagahama et al., FEBS Lett. (1998) 434, 155–159). Although PACE4A is a putative secretory enzyme, it matures and is secreted much slower than general secretory proteins. In this study, we investigated the molecular mechanism underlying this slow maturation. The deletion of 25 amino acids at the carboxy terminus is sufficient for a marked acceleration in both the maturation and secretion of PACE4A. The carboxyl-truncated proPACE4A existed only as a monomer-sized form in the endoplasmic reticulum, whereas the wild type of proPACE4A existed in larger forms. Further, the fusion construct of yellow fluorescent protein and the carboxy-terminal sequence of PACE4A associated with the proPACE4A moiety and inhibited maturation. Thus, the carboxy terminus of PACE4A functions as a potent autoinhibitor of its activation, resulting in the retention of proPACE4A in the endoplasmic reticulum. These findings indicate that PACE4A activity is highly controlled by a unique system at post-translational level. [from: Taniguchi T., Kuroda R., Sakurai K., Nagahama M., Wada I., Tsuji A., Matsuda Y. A Critical Role for the Carboxy Terminal Region of the Proprotein Convertase, PACE4A, in the Regulation of Its Autocatalytic Activation Coupled with Secretion (2002) Biochemical and Biophysical Research Communications 290(2) 878-884.] Source: Professor Akihiko Tsuji, Faculty of Engineering, Tokushima University. |
| Host | Rabbit |
| Species specificity | HU |
| Product name | Anti Inter-Alpha-Trypsin Inhibitor Heavy Chain H4 (ITIH4) pAb (Brown Norway Rat, Antiserum) |
| Cat No | CAC-ICA-TG2-RTP1 |
| Description | The inter-alpha-trypsin inhibitors (ITI) are a family of structurally related plasma serine protease inhibitors involved in extracellular matrix stabilization and in prevention of tumor metastasis. The ITI family contains multiple proteins made up of a light chain and a variable number of heavy chains (Salier et al., 1987; Himmelfarb et al., 2004). Peptides derived from the proline-rich potentially active peptide may be biomarkers for a variety of disease states including breast cancer. |
| Host | RT |
| Species specificity | HU RT |
| Figure 1 | 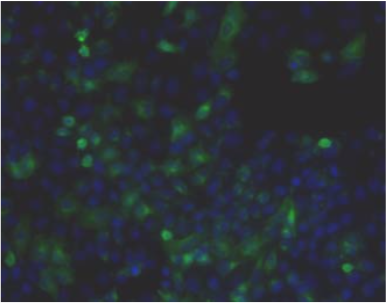 |
| Immunohistochemistry. Hela cells ICAFectin441-transfected with a plasmid encoding human ITIH4 were fixed, permeabilized and stained with rat anti-human ITIH4 serum (diluted 1/50) and Alexa488 goat anti rat IgG secondary antibody. Nuclei were counterstained with DAPI |
|
| Figure 2 | 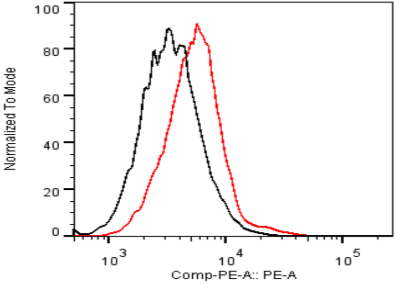 |
| Flow cytometry. Untransfected (black) and transfected with ICAfectin 441 and plasmid encoding human ITIH4 (red) Hela cells were fixed, permeabilized and stained with rat anti-human ITIH4 Polyclonal Antibodies diluted 1/100 and R-PE goat anti rat IgG secondary antibody |
|
| Figure 3 | 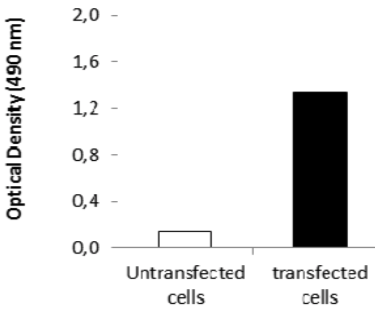 |
| ELISA. Lysates of HeLa cells untransfected (red) and ICAFectin441-transfected with a plasmid encoding human ITIH4 were coated in 96 wells plate. Wells were stained with rat anti-human ITIH4 serum and HRP goat anti rat IgG secondary antibody |
|
| Product name | Anti er-Alpha-Trypsin Inhibitor Heavy Chain H4 (ITIH4) pAb (Sprague Dawley Rat, Antiserum) |
| Cat No | CAC-ICA-TG2-RTP2 |
| Description | The inter-alpha-trypsin inhibitors (ITI) are a family of structurally related plasma serine protease inhibitors involved in extracellular matrix stabilization and in prevention of tumor metastasis. The ITI family contains multiple proteins made up of a light chain and a variable number of heavy chains (Salier et al., 1987; Himmelfarb et al., 2004). Peptides derived from the proline-rich potentially active peptide may be biomarkers for a variety of disease states including breast cancer. |
| Host | RT |
| Species specificity | HU RT |
| Figure 1 | 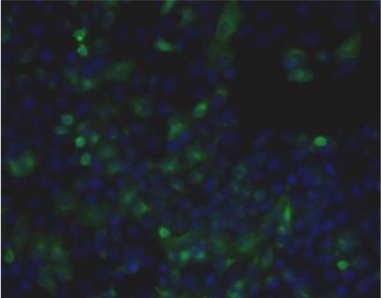 |
| Immunohistochemistry. Hela cells ICAFectin441-transfected with a plasmid encoding human ITIH4 were fixed, permeabilized and stained with rat anti-human ITIH4 serum (diluted 1/50) and Alexa488 goat anti rat IgG secondary antibody. Nuclei were counterstained with DAPI. |
|
| Figure 2 | 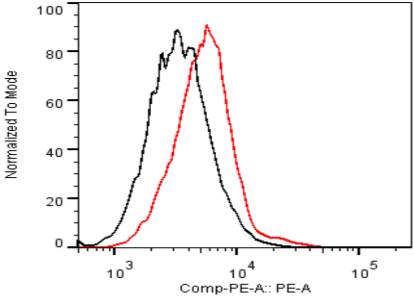 |
| Flow Cytometry. HeLa cells untransfected (black) and ICAFectin441-transfected with a plasmid encoding human ITIH4 (red) were stained with rat anti-human ITIH4 serum (diluted 1/100) and R-PE goat anti rat IgG secondary antibody. |
|
| Figure 3 | 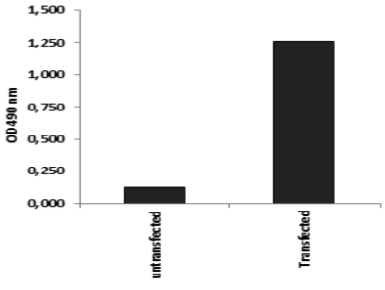 |
| ELISA. Lysates of HeLa cells untransfected (red) and ICAFectin441-transfected with a plasmid encoding human ITIH4 were coated in 96 wells plate. Wells were stained with rat anti-human ITIH4 serum and HRP goat anti rat IgG secondary antibody. |
|
| Cell adhesion molecule and hemidesmosome-related |
|
| Hemidesmosomes are adhesive structures between cells and the extracellular matrix. They play a role in anchoring intermediate fibers to the extracellular basement membrane. Structurally, hemidesmosomes occur in two forms: Type I and Type II. Type I hemidesmosomes develop in stratified epithelia such as the epidermis. Its main components include the intracellular linker proteins Plectin and BPAG1, the adhesion receptor integrin α6β4 and collagen type BP180/XVII. Type II hemidesmosomes occur in blood vessels, Schwann cells, and digestive tract epithelia as a simplified form of Type I hemidesmosomes, consisting only plectin and integrin α6β4. The hemidesmosomal adhesion receptor is normally associated with Laminin 5 in the basement membrane. Furthermore, Laminin 5 (of which Laminin gamma 2 is a subunit) is linked to collagen fibers in the dermis via type VII collagen. Genetic deletion of hemidesmosome-related proteins causes various forms of epidermolysis bullosa, highlighting their importance in promoting adhesion between the epidermis and the basement membrane. | |
| Product name | Anti Plectin (PCN/PLTN) mAb (Clone PN753) |
| Cat No | CAC-NU-01-PLN |
| Description | Plectin interlinks intermediate filaments with microtubules and microfilaments and anchors intermediate filaments to desmosomes or hemidesmosomes. Could also bind muscle proteins such as actin to membrane complexes in muscle. May be involved not only in the filaments network, but also in the regulation of their dynamics. Structural component of muscle. Isoform 9 plays a major role in the maintenance of myofiber integrity. References: 1) Yamauchi T., et al. J. Dermatol. Sci., 76:25-33 (2014). 2) Hirako Y., et al. Exp. Cell Res., 324:172-182 (2014). 3) Hirako Y., et al. J. Baiol. Chem., 273:9711-9717 (1998). |
| Host | Mouse |
| Species specificity | HU RT RAB POR |
| Figure 1 | 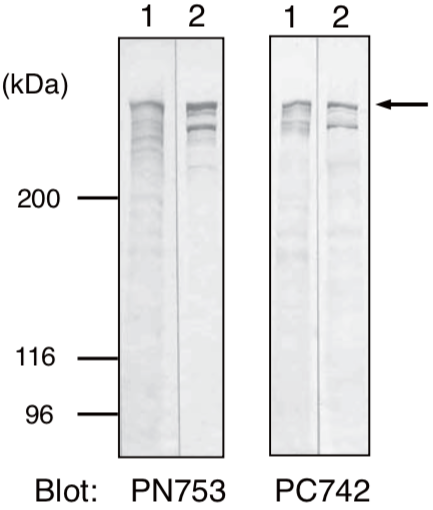 |
| Immunoblot analysis. Whole cell lysates prepared from DJM-1 cells (lane 1) and HeLa cells (lane 2) were separated by SDS-PAGE (5% gel) and immunoblotted with PN753 or PC742 (1: 200 dilution). Plectin antibodies detected approximately 500 kDa sized bands (arrow). The smaller polypeptide found in lane 2 may be a degraded product or alternatively spliced rod-less isoform of plectin. |
|
| Figure 2 |  |
| Locations of epitopes for Plectin antibodies. Clones PN753 and PC742 were obtained by immunizing mice with the NH2- (173-595 aa) or the COOH-terminal (2,930-3,153 aa) regions of human Plectin (4,574 aa). Grey box represents a predicted coiled-coil region (1,300-2,600 aa). |
|
| Figure 3 | 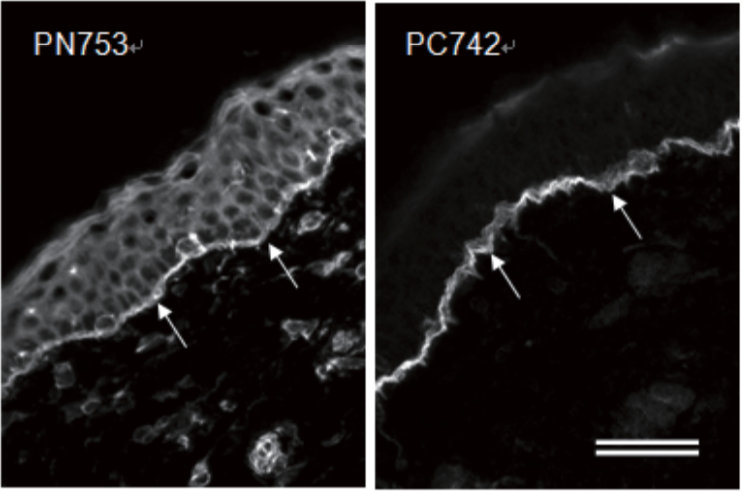 |
| Immunofluorescence microscopy of human skin. Human skin sections were stained with PC742 (1:100 dilution) or PN753 (1:100 dilution). Arrows indicate dermal-epidermal junctions. PN753 stains epidermal cells in addition to hemidesmosomes at the dermal-epidermal junction. Section were fixed with -20°C acetone for 10 min. Bar: 50 um. |
|
| Product name | Anti Laminin Subunit Gamma-2 mAb (Clone YN557) |
| Cat No | CAC-NU-01-LA2 |
| Description | The Laminin gamma 2 chain is highly homologous to the gamma 1 chain; however, it lacks domain VI, and domains V, IV and III are shorter. It is expressed in several fetal tissues but differently from gamma 1, and is specifically localized to epithelial cells in skin, lung and kidney. The gamma 2 chain together with alpha 3 and beta 3 chains constitute laminin 5 (earlier known as kalinin), which is an integral part of the anchoring filaments that connect epithelial cells to the underlying basement membrane. The epithelium-specific expression of the gamma 2 chain implied its role as an epithelium attachment molecule, and mutations in this gene have been associated with junctional epidermolysis bullosa, a skin disease characterized by blisters due to disruption of the epidermal-dermal junction. |
| Host | Mouse |
| Species specificity | HU BOV |
| Figure 1 | 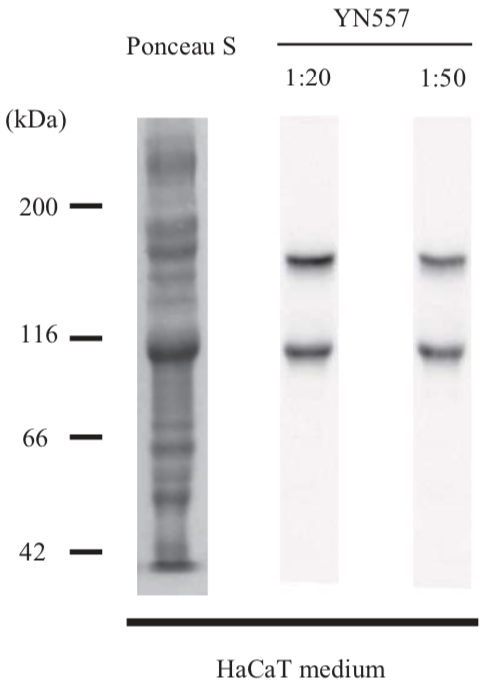 |
| Immunoblot analysis. Concentrated conditioned medium prepared from HaCaT cells was immunoblotted with YN557 antibody (1:20 and 1:50 dilutions). Conditioned medium was concentrated by ammonia sulfate precipitation as described previously (ref. 2). YN557 antibody detected 105-kDa and 155 kDa bands for processed and non-processed form of laminin gamma 2, respectively (ref. 1). |
|
| Figure 2 | 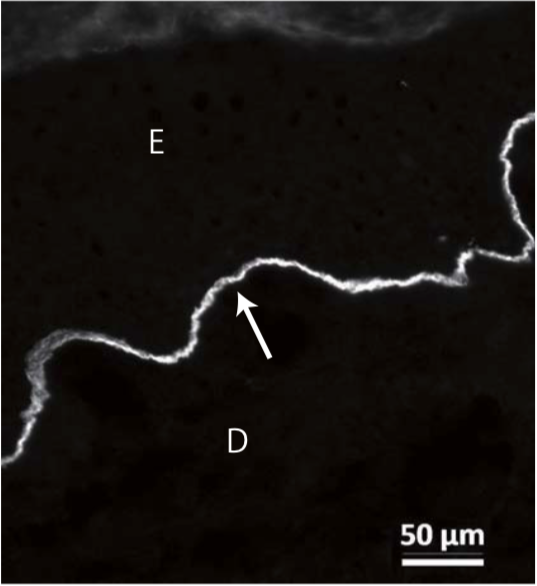 |
| Immunofluorescence microscopy of human skin. A human skin section was stained with YN557 antibody at 1:200 dilution. The antibody revealed the location of laminin gamma 2 chain at the dermal-epidermal junction (arrow). Frozen sections were prepared as described previously (ref. 2). E: epidermis, D: dermis. Bar = 50 um. |
|
| Product name | Anti Collagen Alpha-1(XVII) Chain (BP180/BPAG2) mAb (Clone C34) |
| Cat No | CAC-NU-01-BP2 |
| Description | Unlike most collagens, collagen XVII is a transmembrane protein. Collagen XVII is a structural component of hemidesmosomes, multiprotein complexes at the dermal-epidermal basement membrane zone that mediate adhesion of keratinocytes to the underlying membrane. Mutations in this gene are associated with both generalized atrophic benign and junctional epidermolysis bullosa. |
| Host | Mouse |
| Species specificity | HU |
| Figure 1 | 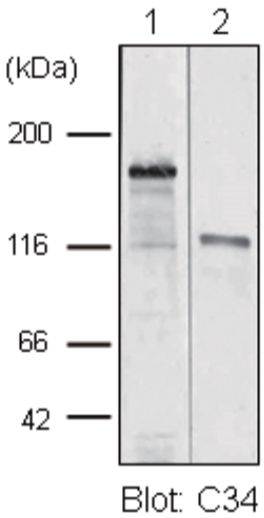 |
| Immunoblot analysis. TritonX-100 insoluble cytoskeletal fraction (lane 1) and concentrated conditioned medium (lane 2) prepared from DJM-1 cells were separated by SDS-PAGE (7.5% gel) and immunoblotted with the C34 antibody (1:200 dilution). The C34 antibody detected a band at approximately 180 kDa in lane 1. This antibody also reacted with a 120-kDa shed ectodomain of BP180 in lane 2. |
|
| Figure 2 | 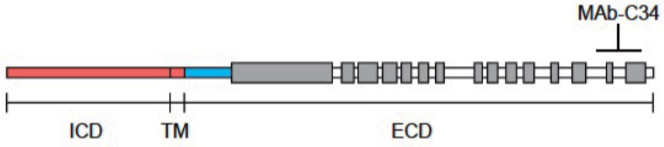 |
| Location of the epitope for the C34 antibody. The C34 antibody does not react with the 100-kDa extracellular fragment of BP180, which lacks the COOH-terminal portion (ref. 1). The result indicates that the C34 antibody recognizes an epitope located at the COOH-terminal portion of about 20 kDa. ICD, TM and ECD refer to intracellular, transmembrane and extracellular domains. Collagenous domains are shown by gray boxes. |
|
| Figure 3 |  |
| Immunofluorescence microscopy of human skin. A human skin section was stained with C34 antibody at 1:200 dilution. The antibody revealed the location of BP180 molecules at the dermal-epidermal junction (arrow). E: epidermis, D: dermis. Bar = 50 um. Frozen sections were prepared as described previously (ref. 3). |
|
| Product name | Anti Keratin, Type II Cytoskeletal 8 (Keratin-8) mAb (Clone RL273) |
| Cat No | CAC-NU-01-KE8 |
| Description | Keratin 8 typically dimerizes with Keratin 18 to form an intermediate filament in simple single-layered epithelial cells. Keratin 8 plays a role in maintaining cellular structural integrity and also functions in signal transduction and cellular differentiation. Mutations in this gene cause cryptogenic cirrhosis.Together with Keratin 19, helps to link the contractile apparatus to dystrophin at the costameres of striated muscle. |
| Host | Mouse |
| Species specificity | HU RAB |
| Figure 1 | 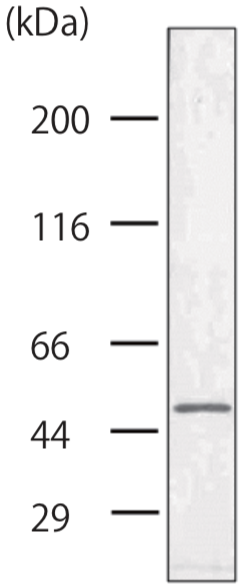 |
| Immunoblot analysis. Whole cell lysate prepared from DLD-1 human colorectal adenocarcinoma cell line was immunoblotted with RL273 antibody (1:200 dilution). The antibody detected a polypeptide band for keratin 8 at approximately 50 kDa. |
|
| Figure 2 | 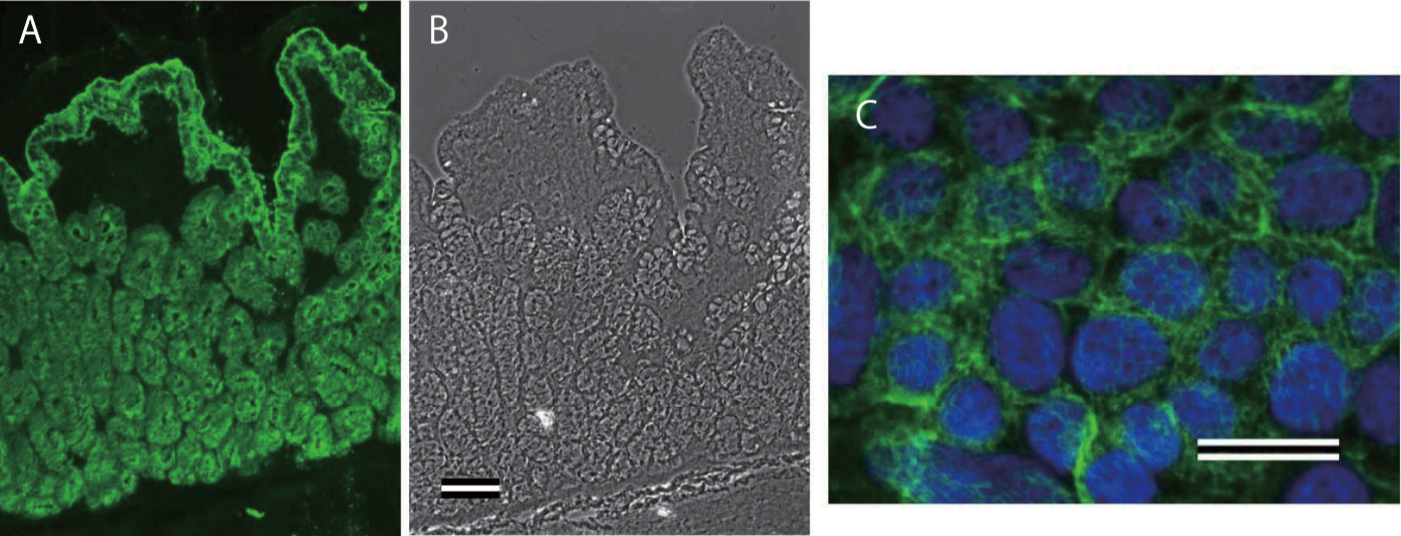 |
| Immunofluorescence microscopy of rabbit colon and DLD-1 cells. Frozen sections of rabbit colon were stained by RL273 antibody at 1:250 dilution (A). The absorptive epithelial cells and mucosal gland cells were stained by the antibody. Panel B shows the phase contrast image of A. Bar = 100 um. Acetone-fixed DLD-1 cells were stained by RL273 antibody at 1:250 dilution (C), showing the filamentous networks of keratins (green). Cell nuclei were visualized by DAPI (blue). Bar = 25 um. |
|
| Product name | Anti Dystonin (BPAG1/BP230) mAb (Clone 279) |
| Cat No | CAC-NU-01-BP1 |
| Description | BPAG1 is a 230-kDa cytoplasmic protein localized to hemidesmosomes of stratified epithelia and is one of the major antigenic proteins of bullous pemphigoid (BP), a chronic autoimmune skin disease. This anti-BPAG1 monoclonal antibody (clone 279) was raised against bovine BPAG1 and can be used in western blotting and immunofluorescent staining (IF) in multiple animal species, including humans and mice. |
| Host | Mouse |
| Species specificity | HU RT BOV RAB POR |
| Figure 1 | 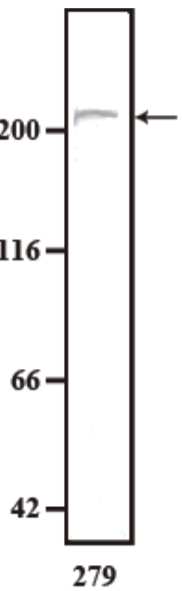 |
| Immunoblot analysis. Whole cell lysate prepared from DJM-1 cells was immunoblotted with mAb 279 at 1:100 dilution. mAb 279 recognized BPAG1 (Bullous Pemphigoid antigen 1) as a band at approximately 230 kDa (arrow). |
|
| Figure 2 | 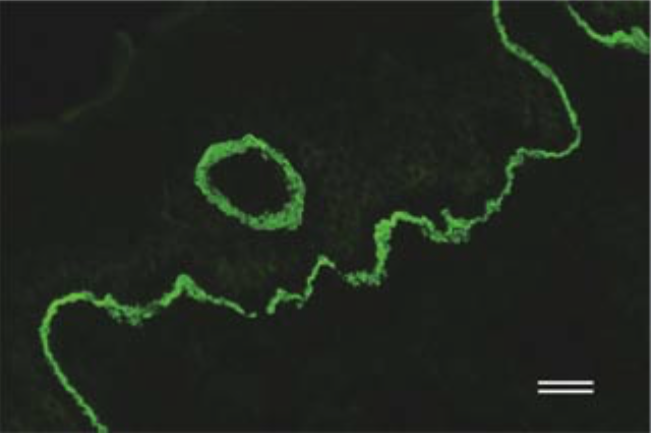 |
| Immunofluorescence microscopy of human skin. Human skin was stained by mAb 279 (1:50), showing that BPAG1 localized at the dermal -epidermal junction (green). Bar: 40 um. |
|
| Figure 3 | 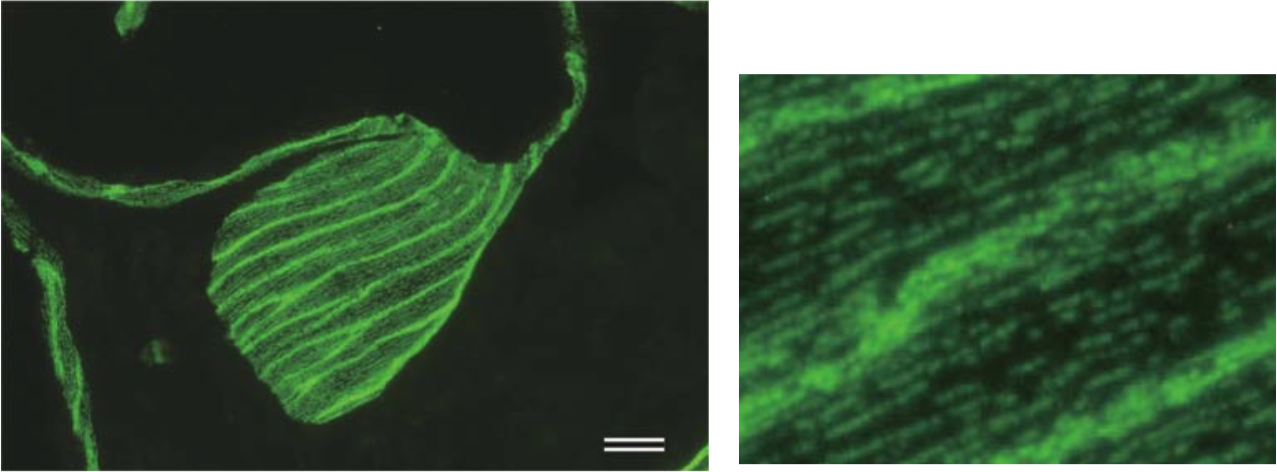 |
| Immunofluorescence microscopy of bovine skin. Bovine skin was stained by mAb 279 (1:50 dilution). Myoepithelial cells of skin apocrine glands were demonstrated to express BPAG1 (left panel, green). The close-up image (right) shows dotted lines of hemidesmosomes, which are cell to substrate adhesion structures containing BPAG1. Scale bar: 20 um. |
|
| Product name | Anti Epithelial Cell Adhesion Molecule (EPCAM) mAb (Clone hrk29) |
| Cat No | CAC-HT-MAB1 |
| Description | Epithelial cell adhesion molecule (EpCAM, CD326) is a glycoprotein of ∼40 kd that was initially described as a tumor-associated antigen by Koprowski and colleagues in 1979. It is of particular interest because of its high level of expression on a variety of carcinomas. Thus, it comes as no surprise that EpCAM is a candidate protein for tumor diagnosis and therapy. Most studies have focused on EpCAM as a favorable target for tumor therapy, involving monoclonal and bi-/tri-specific antibodies, vaccination strategies, toxin-conjugated antibody fragments, and an antibody fragment-targeted sTRAIL fusion protein. In contrast to the broad expression pattern of most other CAMs in normal tissues, the expression of EpCAM is restricted to normal epithelial cells. Based on the observation that EpCAM mediates cell-cell adhesion, Litvinov and colleagues proposed that EpCAM is a CAM. However, recent insights revealed a more versatile role for EpCAM, not merely limited to cell adhesion but similar to other CAMs, including processes such as signaling, cell migration, proliferation, and differentiation. [adapted from: Trzpis M, McLaughlin PM, de Leij LM, Harmsen MC. (2007) Epithelial cell adhesion molecule: more than a carcinoma marker and adhesion molecule. Am J Pathol. 171(2):386-95.] |
| Host | MS |
| Species specificity | HU |
| Product name | Anti Integrin Alpha-6 (VLA-6) mAb (Clone 537D5) |
| Cat No | CAC-PRPG-ITG-M01 |
| Description | Integrins are conserved, cation-dependent transmembrane receptors essential for cell survival and growth. They are comprised of α and β subunits that are differentially involved in ligand binding and connection with the cytoskeleton. They link cells to the extracellular matrix (ECM) and to cell surface-bound adhesion molecules, such as to allow cells to properly organize within tissues in relation to underlying and/or surrounding matrices. Thus, in epithelia and vasculature integrins are critical in structuring the intricate junctional complexes with underlying basement membranes, whereas in connective tissues they allow cells to form stable attachments (i.e. focal adhesions) with their surrounding interstitial matrices and rapidly convert (bidirectionally) from stationary to motile phenotypes. Integrins not engaged in ligand binding are generally dispersed on the surface of cells but tend to form microclusters. Upon ligand engagement they reorganize to form larger clusters that permit the stabilization of cell-ECM or cell-cell interactions. Simultaneously, through phosphorylation of the cytoplasmic portion of the β subunit, integrins associate with key cytoskeletal adapter proteins, such as vinculin, talin, paxillin, tensin and FAK to activate complex signal transduction pathways converging with those elicited by growth factor receptors and other receptors for soluble and membrane-bound signal molecules. This results in the activation of the cell cycle, cell differentiation programs and/or the acquisition of motile properties. Conversely, loss of integrin binding to the matrix causes a type of programmed cell death known as anoikis. There are more than 15 α subunits and 8 β subunits, which pair with each other in different combinations to generate a repertoire of over 20 different integrin receptors. These may be selective, binding one or two ligands or promiscuous, binding multiple ligands. Similarly, the same ECM component may be recognized by one individual integrin receptor or multiple receptors. Integrin expression is frequently altered in pathological conditions and mutations in the INTG genes are associated with inheritable diseases. In cancer, integrins are fundamental in conferring a more aggressive behavior to malignant cells and are therefore considered attractive therapeutic targets. However, thus far, only one anti-integrin drug is registered for clinical application and its use is for the treatment of neurological rather neoplastic diseases. The α6 integrin subunit pairs with two distinct β subunits, β1 and β4, and with the latter one it forms a unique integrin receptor that is essential for the assembly and maintenance of hemidesmosomes. There are a total 8 different alternatively-spliced α6 isoforms known which show a diverse tissue distribution, i.e. isoforms containing segment X1 are ubiquitously expressed, whereas isoforms containing segment X1X2 are expressed in heart, kidney, placenta, colon, duodenum, myoblasts and myotubes. Similarly, in some tissues, isoforms containing cytoplasmic segment A and isoforms containing segment B are detected while in others, only isoforms containing one cytoplasmic segment are found. |
| Host | MS |
| Species specificity | HU |
| Figure 1 | 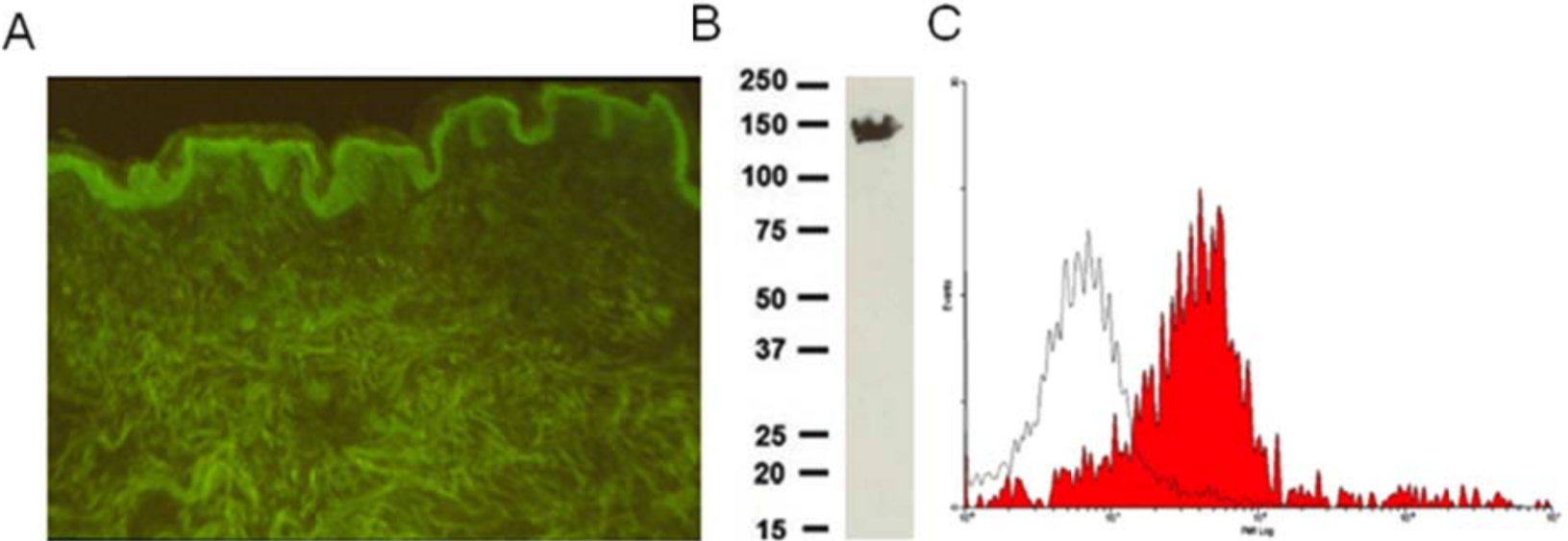 |
| A) Immunolabelling of human adult skin; B) Immunoblot analysis of sarcoma HT1080 whole-cell extracts; C) Flow cytometric analysis of A375 melanoma cells. | |
| Bone and cartilage-related |
|
| Bone is a very dense, specialized form of connective tissue, as different as could be from adipose tissue, even though closely related in origin. Like reinforced concrete, bone matrix is predominantly a mixture of tough fibers (type I collagen fibrils), which resist pulling forces, and solid particles (calcium phosphate as hydroxyapatite crystals), which resist compression. The volume occupied by the collagen is nearly equal to that occupied by the calcium phosphate. The collagen fibrils in adult bone are arranged in regular plywood like layers, with the fibrils in each layer lying parallel to one another but at right angles to the fibrils in the layers on either side. For all its rigidity, bone is by no means a permanent and immutable tissue. Running through the hard extracellular matrix are channels and cavities occupied by living cells, which account for about 15% of the weight of compact bone. These cells are engaged in an unceasing process of remodeling: one class of cells (osteoclasts, related to macrophages) demolishes old bone matrix while another (osteoblasts, related to fibroblasts) deposits new bone matrix. This mechanism provides for continuous turnover and replacement of the matrix in the interior of the bone. Unlike soft tissues, which can grow by internal expansion, bone can grow only by apposition—that is, by the laying down of additional matrix and cells on the free surfaces of existing bone. During development, this process must occur in coordination with the growth of other tissues, in such a way that the pattern of the body can be scaled up without its proportions being radically disturbed. For most of the skeleton, and in particular for the long bones of the limbs and trunk, the coordinated growth is achieved by a complex strategy. A set of minute “scale models” of these bones are first formed out of cartilage. Each scale model then grows, and as new cartilage is formed, the older cartilage is replaced by bone. Cartilage growth and erosion and bone deposition are so ingeniously coordinated during development that the adult bone, though it may be half a meter long, is almost the same shape as the initial cartilaginous model, which was no more than a few millimeters long. Defective growth of cartilage during the development of long bones, as a result of a dominant mutation in the gene that codes for an FGF receptor (FGFR3), is responsible for the commonest form of dwarfism, known as achondroplasia. Conversely, osteoblasts are lacking in individuals with mutations that disrupt production of a gene regulatory protein (called CBFA1) specifically required for osteoblast differentiation: mice homozygous for this genetic defect are born with a skeleton consisting solely of cartilage and die soon after birth as a result. Cartilage is a simple tissue, consisting of cells of a single type—chondrocytes—embedded in a more or less uniform matrix. The cartilage matrix is deformable, and the tissue grows by expanding as the chondrocytes divide and secrete more matrix. Bone is more complex. The bone matrix is secreted by osteoblasts that lie at the surface of the existing matrix and deposit fresh layers of bone onto it. Some of the osteoblasts remain free at the surface, while others gradually become embedded in their own secretion. This freshly formed material (consisting chiefly of type I collagen) is called osteoid. It is rapidly converted into hard bone matrix by the deposition of calcium phosphate crystals in it. Once imprisoned in hard matrix, the original bone-forming cell, now called an osteocyte, has no opportunity to divide, although it continues to secrete further matrix in small quantities around itself. The osteocyte, like the chondrocyte, occupies a small cavity, or lacuna, in the matrix, but unlike the chondrocyte it is not isolated from its fellows. Tiny channels, or canaliculi, radiate from each lacuna and contain cell processes from the resident osteocyte, enabling it to form gap junctions with adjacent osteocytes. Although the networks of osteocytes do not themselves secrete or erode substantial quantities of matrix, they probably play a part in controlling the activities of the cells that do. While bone matrix is deposited by osteoblasts, it is eroded by osteoclasts. These large multinucleated cells originate, like macrophages, from hemopoietic stem cells in the bone marrow. The precursor cells are released as monocytes into the bloodstream and collect at sites of bone resorption, where they fuse to form the multinucleated osteoclasts, which cling to surfaces of the bone matrix and eat it away. Osteoclasts are capable of tunneling deep into the substance of compact bone, forming cavities that are then invaded by other cells. A blood capillary grows down the center of such a tunnel, and the walls of the tunnel become lined with a layer of osteoblasts. To produce the plywood like structure of compact bone, these osteoblasts lay down concentric layers of new matrix, which gradually fill the cavity, leaving only a narrow canal surrounding the new blood vessel. Many of the osteoblasts become trapped in the bone matrix and survive as concentric rings of osteocytes. At the same time as some tunnels are filling up with bone, others are being bored by osteoclasts, cutting through older concentric systems. The consequences of this perpetual remodeling are beautifully displayed in the layered patterns of matrix observed in compact bone. Through remodeling, bones are endowed with a remarkable ability to adjust their structure in response to long-term variations in the load imposed on them. This adaptive behavior implies that the deposition and erosion of the matrix are somehow controlled by local mechanical stresses, but the mechanisms involved are not understood. The bone cells secrete signal proteins that become trapped in the matrix, and it is likely that these are released when the matrix is degraded or suitably stressed. The released proteins, especially members of the BMP subfamily of TGFβ proteins, may help to guide the remodeling process. Remodeling carries a risk: defects in its control can lead to osteoporosis, where there is excessive erosion of the bone matrix and weakening of the bone, or to the opposite condition, osteopetrosis, where the bone becomes excessively thick and dense. The replacement of cartilage by bone in the course of development is also thought to depend on the activities of osteoclasts. As the cartilage matures, its cells in certain regions become greatly enlarged at the expense of the surrounding matrix, and the matrix itself becomes mineralized, like bone, by the deposition of calcium phosphate crystals. The swollen chondrocytes die, leaving large empty cavities. Osteoclasts and blood vessels invade the cavities and erode the residual cartilage matrix, while osteoblasts following in their wake begin to deposit bone matrix. The only surviving remnant of cartilage in the adult long bone is a thin layer that forms a smooth covering on the bone surfaces at joints, where one bone articulates with another (22-56). Some cells capable of forming new cartilage persist, however, in the connective tissue that surrounds a bone. If the bone is broken, the cells in the neighborhood of the fracture repair it by a sort of recapitulation of the original embryonic process: cartilage is first laid down to bridge the gap and is then replaced by bone. The capacity for self-repair, so strikingly illustrated by the tissues of the skeleton, is a property of living structures that has no parallel among present-day man-made objects. [from: Alberts B, Johnson A, Lewis J, et al. Molecular Biology of the Cell. 4th edition. New York: Garland Science; 2002. Available from:https://www.ncbi.nlm.nih.gov/books/NBK21054/] |
|
| Product name | Anti Chondromodulin-I (ChM-I) mAb (Clone hCHM-1) |
| Cat No | CAC-TCS-001 |
| Description | Lect1 encodes a glycosylated transmembrane protein that is cleaved to form a mature, secreted protein. The N-terminus of the precursor protein shares characteristics with other surfactant proteins and is sometimes called chondrosurfactant protein, although no biological activity has yet been defined for it. The C-terminus of the precursor protein contains a 25 kDa mature protein called leukocyte cell-derived chemotaxin-1 or chondromodulin-1. The mature protein promotes chondrocyte growth and inhibits angiogenesis. This gene is expressed in the avascular zone of prehypertrophic cartilage, and its expression decreases during chondrocyte hypertrophy and vascular invasion. The mature protein likely plays a role in endochondral bone development by permitting cartilaginous anlagen to be vascularized and replaced by bone. It may also be involved in the broad control of tissue vascularization during development. Alternative splicing results in multiple transcript variants encoding different isoforms.[from: Wikipedia contributors. (2017, October 27). LECT1. In Wikipedia, The Free Encyclopedia. Retrieved 17:42, June 3, 2019, from https://en.wikipedia.org/w/index.php?title=LECT1&oldid=807301938] |
| Host | MS |
| Species specificity | HU MS RT BOV |
| Figure 1 | 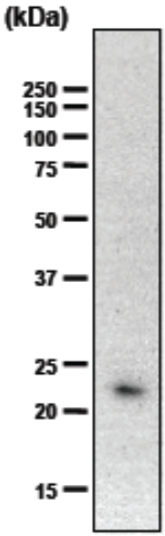 |
| Recombinant human ChM-1 (5 ng) was subjected to immunoblot analysis using hChM-1 antibody at 1/100 dilution. | |
| Product name | Anti Chondromodulin-I (ChM-I) mAb (Clone hCHM-2) |
| Cat No | CAC-TCS-002 |
| Description | Lect1 encodes a glycosylated transmembrane protein that is cleaved to form a mature, secreted protein. The N-terminus of the precursor protein shares characteristics with other surfactant proteins and is sometimes called chondrosurfactant protein, although no biological activity has yet been defined for it. The C-terminus of the precursor protein contains a 25 kDa mature protein called leukocyte cell-derived chemotaxin-1 or chondromodulin-1. The mature protein promotes chondrocyte growth and inhibits angiogenesis. This gene is expressed in the avascular zone of prehypertrophic cartilage, and its expression decreases during chondrocyte hypertrophy and vascular invasion. The mature protein likely plays a role in endochondral bone development by permitting cartilaginous anlagen to be vascularized and replaced by bone. It may also be involved in the broad control of tissue vascularization during development. Alternative splicing results in multiple transcript variants encoding different isoforms. [from: Wikipedia contributors. (2017, October 27). LECT1. In Wikipedia, The Free Encyclopedia. Retrieved 17:42, June 3, 2019, from https://en.wikipedia.org/w/index.php?title=LECT1&oldid=807301938] |
| Host | MS |
| Species specificity | HU |
| Figure 1 |  |
| Recombinant human ChM-1 (5 ng) was subjected to immunoblot analysis using hChM-2 antibody at 1/1000 dilution. | |
| Product name | Anti Chondromodulin-I (ChM-I) mAb (Clone hCHM-3) |
| Cat No | CAC-TCS-003 |
| Description | Lect1 encodes a glycosylated transmembrane protein that is cleaved to form a mature, secreted protein. The N-terminus of the precursor protein shares characteristics with other surfactant proteins and is sometimes called chondrosurfactant protein, although no biological activity has yet been defined for it. The C-terminus of the precursor protein contains a 25 kDa mature protein called leukocyte cell-derived chemotaxin-1 or chondromodulin-1. The mature protein promotes chondrocyte growth and inhibits angiogenesis. This gene is expressed in the avascular zone of prehypertrophic cartilage, and its expression decreases during chondrocyte hypertrophy and vascular invasion. The mature protein likely plays a role in endochondral bone development by permitting cartilaginous anlagen to be vascularized and replaced by bone. It may also be involved in the broad control of tissue vascularization during development. Alternative splicing results in multiple transcript variants encoding different isoforms. [from: Wikipedia contributors. (2017, October 27). LECT1. In Wikipedia, The Free Encyclopedia. Retrieved 17:42, June 3, 2019, from https://en.wikipedia.org/w/index.php?title=LECT1&oldid=807301938] |
| Host | MS |
| Species specificity | HU MS RT BOV |
| Figure 1 | 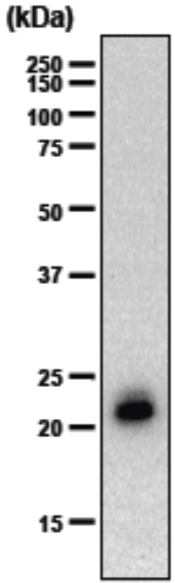 |
| Recombinant human ChM-1 (5 ng) was subjected to immunoblot analysis using hChM-3 antibody at 1/1000 dilution. | |
| Product name | Anti Chondromodulin-I (ChM-I) mAb (Clone hCHM-4) |
| Cat No | CAC-TCS-004 |
| Description |
Lect1 encodes a glycosylated transmembrane protein that is cleaved to form a mature, secreted protein. The N-terminus of the precursor protein shares characteristics with other surfactant proteins and is sometimes called chondrosurfactant protein, although no biological activity has yet been defined for it. The C-terminus of the precursor protein contains a 25 kDa mature protein called leukocyte cell-derived chemotaxin-1 or chondromodulin-1. The mature protein promotes chondrocyte growth and inhibits angiogenesis. This gene is expressed in the avascular zone of prehypertrophic cartilage, and its expression decreases during chondrocyte hypertrophy and vascular invasion. The mature protein likely plays a role in endochondral bone development by permitting cartilaginous anlagen to be vascularized and replaced by bone. It may also be involved in the broad control of tissue vascularization during development. Alternative splicing results in multiple transcript variants encoding different isoforms. [from: Wikipedia contributors. (2017, October 27). LECT1. In Wikipedia, The Free Encyclopedia. Retrieved 17:42, June 3, 2019, from https://en.wikipedia.org/w/index.php?title=LECT1&oldid=807301938] |
| Host | MS |
| Species specificity | HU MS RT BOV |
| Figure 1 | 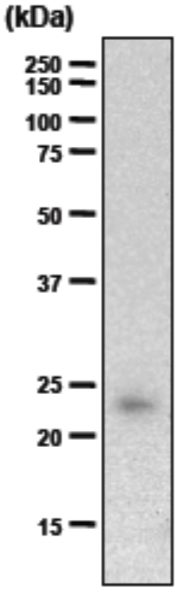 |
| Recombinant human ChM-1 (5 ng) was subjected to immunoblot analysis using hChM-3 antibody at 1/500 dilution. | |
| Product name | Anti Chondromodulin-I (ChM-I) mAb (Clone hCHM-5) |
| Cat No | CAC-TCS-005 |
| Description | Lect1 encodes a glycosylated transmembrane protein that is cleaved to form a mature, secreted protein. The N-terminus of the precursor protein shares characteristics with other surfactant proteins and is sometimes called chondrosurfactant protein, although no biological activity has yet been defined for it. The C-terminus of the precursor protein contains a 25 kDa mature protein called leukocyte cell-derived chemotaxin-1 or chondromodulin-1. The mature protein promotes chondrocyte growth and inhibits angiogenesis. This gene is expressed in the avascular zone of prehypertrophic cartilage, and its expression decreases during chondrocyte hypertrophy and vascular invasion. The mature protein likely plays a role in endochondral bone development by permitting cartilaginous anlagen to be vascularized and replaced by bone. It may also be involved in the broad control of tissue vascularization during development. Alternative splicing results in multiple transcript variants encoding different isoforms. [from: Wikipedia contributors. (2017, October 27). LECT1. In Wikipedia, The Free Encyclopedia. Retrieved 17:42, June 3, 2019, from https://en.wikipedia.org/w/index.php?title=LECT1&oldid=807301938] |
| Host | MS |
| Species specificity | HU MS RT BOV |
| Figure 1 | 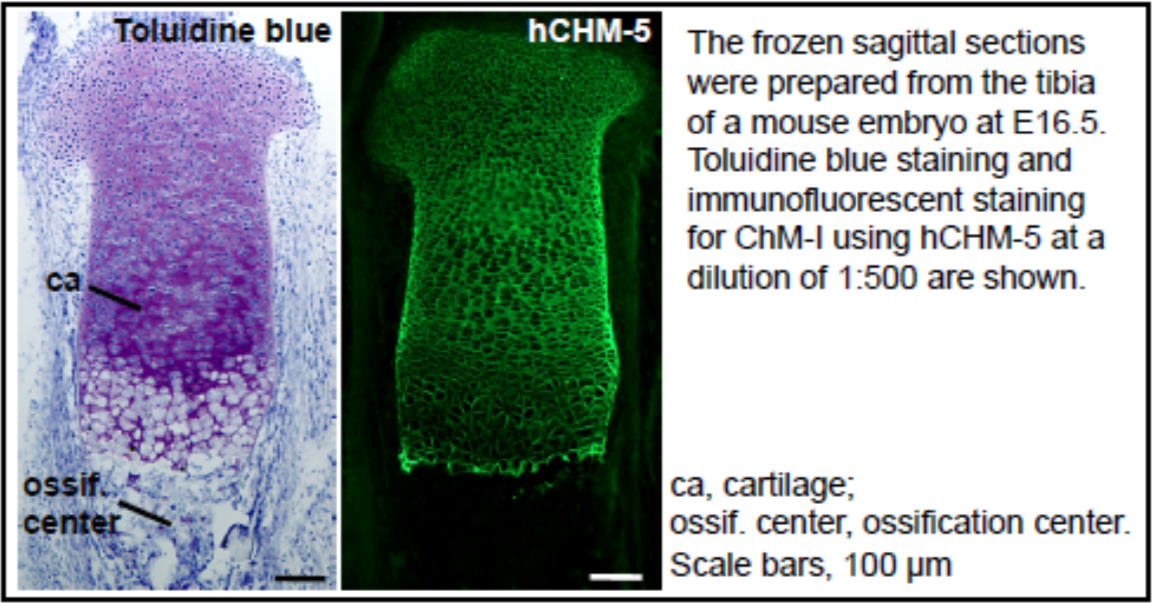 |
| Immunostaining of mouse cartilage. | |
| Product name | Anti Chondromodulin-I (ChM-I) mAb (Clone bCHM-6) |
| Cat No | CAC-TCS-006 |
| Description | Lect1 encodes a glycosylated transmembrane protein that is cleaved to form a mature, secreted protein. The N-terminus of the precursor protein shares characteristics with other surfactant proteins and is sometimes called chondrosurfactant protein, although no biological activity has yet been defined for it. The C-terminus of the precursor protein contains a 25 kDa mature protein called leukocyte cell-derived chemotaxin-1 or chondromodulin-1. The mature protein promotes chondrocyte growth and inhibits angiogenesis. This gene is expressed in the avascular zone of prehypertrophic cartilage, and its expression decreases during chondrocyte hypertrophy and vascular invasion. The mature protein likely plays a role in endochondral bone development by permitting cartilaginous anlagen to be vascularized and replaced by bone. It may also be involved in the broad control of tissue vascularization during development. Alternative splicing results in multiple transcript variants encoding different isoforms. [from: Wikipedia contributors. (2017, October 27). LECT1. In Wikipedia, The Free Encyclopedia. Retrieved 17:42, June 3, 2019, from https://en.wikipedia.org/w/index.php?title=LECT1&oldid=807301938] |
| Host | MS |
| Species specificity | HU MS RT BOV |
| Figure 1 | 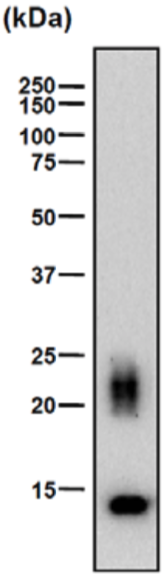 |
| Immunoblot analysis. Rib cartilage extract (2.5 μg protein) from 4-week-old rat was subjected to immunoblot analysis using bChM-6 antibody at 500-fold dilution. ChM-1 is detected as a diffuse band of 20-25 kDa due to variation in glycosylation. This antibody also detects the N-terminal glycosylation domain-deleted form of ChM-1(14 kDa ChM-1). | |
| Product name | Anti Chondromodulin-I (ChM-I) mAb (Clone bCHM-7) |
| Cat No | CAC-TCS-007 |
| Description | Lect1 encodes a glycosylated transmembrane protein that is cleaved to form a mature, secreted protein. The N-terminus of the precursor protein shares characteristics with other surfactant proteins and is sometimes called chondrosurfactant protein, although no biological activity has yet been defined for it. The C-terminus of the precursor protein contains a 25 kDa mature protein called leukocyte cell-derived chemotaxin-1 or chondromodulin-1. The mature protein promotes chondrocyte growth and inhibits angiogenesis. This gene is expressed in the avascular zone of prehypertrophic cartilage, and its expression decreases during chondrocyte hypertrophy and vascular invasion. The mature protein likely plays a role in endochondral bone development by permitting cartilaginous anlagen to be vascularized and replaced by bone. It may also be involved in the broad control of tissue vascularization during development. Alternative splicing results in multiple transcript variants encoding different isoforms. [from: Wikipedia contributors. (2017, October 27). LECT1. In Wikipedia, The Free Encyclopedia. Retrieved 17:42, June 3, 2019, from https://en.wikipedia.org/w/index.php?title=LECT1&oldid=807301938] |
| Host | MS |
| Species specificity | HU MS RT BOV |
| Figure 1 | 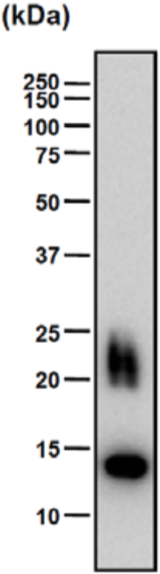 |
| Immunoblot analysis. Rib cartilage extract (2.5 μg protein) from 4-week-old rat was subjected to immunoblot analysis using bChM-7 antibody at 500-fold dilution. ChM-1 is detected as a diffuse band of 20-25 kDa due to variation in glycosylation. This antibody also detects the N-terminal glycosylation domain-deleted form of ChM-1(14 kDa ChM-1). | |
| Product name | Anti Chondromodulin-I (ChM-I) mAb (Clone bCHM-8) |
| Cat No | CAC-TCS-008 |
| Description | Lect1 encodes a glycosylated transmembrane protein that is cleaved to form a mature, secreted protein. The N-terminus of the precursor protein shares characteristics with other surfactant proteins and is sometimes called chondrosurfactant protein, although no biological activity has yet been defined for it. The C-terminus of the precursor protein contains a 25 kDa mature protein called leukocyte cell-derived chemotaxin-1 or chondromodulin-1. The mature protein promotes chondrocyte growth and inhibits angiogenesis. This gene is expressed in the avascular zone of prehypertrophic cartilage, and its expression decreases during chondrocyte hypertrophy and vascular invasion. The mature protein likely plays a role in endochondral bone development by permitting cartilaginous anlagen to be vascularized and replaced by bone. It may also be involved in the broad control of tissue vascularization during development. Alternative splicing results in multiple transcript variants encoding different isoforms. [from: Wikipedia contributors. (2017, October 27). LECT1. In Wikipedia, The Free Encyclopedia. Retrieved 17:42, June 3, 2019, from https://en.wikipedia.org/w/index.php?title=LECT1&oldid=807301938] |
| Host | MS |
| Species specificity | HU MS RT BOV |
| Figure 1 | 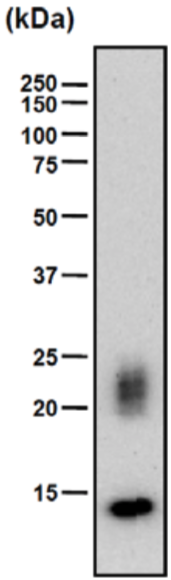 |
| Immunoblot analysis. Rib cartilage extract (2.5 μg protein) from 4-week-old rat was subjected to immunoblot analysis using bChM-8 antibody at 500-fold dilution. ChM-1 is detected as a diffuse band of 20-25 kDa due to variation in glycosylation. This antibody also detects the N-terminal glycosylation domain-deleted form of ChM-1(14 kDa ChM-1). | |
| Product name | Anti Chondromodulin-I (ChM-I) mAb (Clone bCHM-9) |
| Cat No | CAC-TCS-009 |
| Description | Lect1 encodes a glycosylated transmembrane protein that is cleaved to form a mature, secreted protein. The N-terminus of the precursor protein shares characteristics with other surfactant proteins and is sometimes called chondrosurfactant protein, although no biological activity has yet been defined for it. The C-terminus of the precursor protein contains a 25 kDa mature protein called leukocyte cell-derived chemotaxin-1 or chondromodulin-1. The mature protein promotes chondrocyte growth and inhibits angiogenesis. This gene is expressed in the avascular zone of prehypertrophic cartilage, and its expression decreases during chondrocyte hypertrophy and vascular invasion. The mature protein likely plays a role in endochondral bone development by permitting cartilaginous anlagen to be vascularized and replaced by bone. It may also be involved in the broad control of tissue vascularization during development. Alternative splicing results in multiple transcript variants encoding different isoforms. [from: Wikipedia contributors. (2017, October 27). LECT1. In Wikipedia, The Free Encyclopedia. Retrieved 17:42, June 3, 2019, from https://en.wikipedia.org/w/index.php?title=LECT1&oldid=807301938] |
| Host | MS |
| Species specificity | HU MS RT BOV |
| Figure 1 | 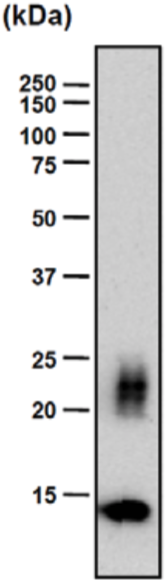 |
| Immunoblot analysis. Rib cartilage extract (2.5 μg protein) from 4-week-old rat was subjected to immunoblot analysis using bChM-9 antibody at 500-fold dilution. ChM-1 is detected as a diffuse band of 20-25 kDa due to variation in glycosylation. This antibody also detects the N-terminal glycosylation domain-deleted form of ChM-1(14 kDa ChM-1). | |
| Product name | Anti Chondromodulin-I (ChM-I) mAb (Clone bCHM-10) |
| Cat No | CAC-TCS-010 |
| Description | Lect1 encodes a glycosylated transmembrane protein that is cleaved to form a mature, secreted protein. The N-terminus of the precursor protein shares characteristics with other surfactant proteins and is sometimes called chondrosurfactant protein, although no biological activity has yet been defined for it. The C-terminus of the precursor protein contains a 25 kDa mature protein called leukocyte cell-derived chemotaxin-1 or chondromodulin-1. The mature protein promotes chondrocyte growth and inhibits angiogenesis. This gene is expressed in the avascular zone of prehypertrophic cartilage, and its expression decreases during chondrocyte hypertrophy and vascular invasion. The mature protein likely plays a role in endochondral bone development by permitting cartilaginous anlagen to be vascularized and replaced by bone. It may also be involved in the broad control of tissue vascularization during development. Alternative splicing results in multiple transcript variants encoding different isoforms. [from: Wikipedia contributors. (2017, October 27). LECT1. In Wikipedia, The Free Encyclopedia. Retrieved 17:42, June 3, 2019, from https://en.wikipedia.org/w/index.php?title=LECT1&oldid=807301938] |
| Host | MS |
| Species specificity | HU MS RT BOV |
| Figure 1 | 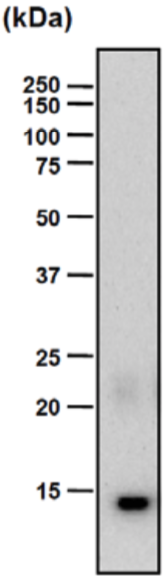 |
| Immunoblot analysis. Rib cartilage extract (2.5 μg protein) from 4-week-old rat was subjected to immunoblot analysis using bChM-10 antibody at 500-fold dilution. ChM-1 is detected as a diffuse band of 20-25 kDa due to variation in glycosylation. This antibody also detects the N-terminal glycosylation domain-deleted form of ChM-1(14 kDa ChM-1). | |
| Product name | Anti Human Transmembrane Glycoprotein NMB (GPNMB) pAb (Rabbit, Purified Ig) |
| Cat No | CAC-ICA-TG1-RBP1 |
| Description | Originally described in melanoma cells, glycoprotein nonmetastatic melanoma B (Gpnmb), also known as osteoactivin and dendritic cell-associated heparin sulfate proteoglycan-dependent integrin ligand, is a heavily N-glycosylated type I transmembrane domain protein with a short cytoplasmic domain containing an endosomal sorting motif. Gpnmb is expressed in numerous cell types, including dendritic cells, macrophages, retinal pigment epithelial cells, osteoblasts and osteoclasts. Recent studies have demonstrated that Gpnmb expressed in macrophages functions as a feedback regulator of proinflammatory responses, and that binding of Gpnmb on antigen-presenting cells to syndecan-4 on activated T cells inhibits T-cell activation. Thus, Gpnmb expressed on antigen-presenting cells may negatively regulate inflammation. Additionally, Gpnmb is involved in osteoblast maturation, and is also associated with motor neuron survival. We previously isolated the gene encoding Gpnmb and demonstrated that it was differentially expressed in the livers of rats fed a choline-deficient, L-amino acid-defined diet. We also found that transgenic Gpnmb expression attenuates the development of hepatic fibrosis. Furthermore, a recent report has demonstrated that Gpnmb is expressed in macrophages infiltrating into injured liver tissues. These findings suggest a pathophysiologic role for Gpnmb in various disorders, including liver injury. However, the specific nature of Gpnmb involvement in liver injury remains unknown. In this study, we focused on the role of infiltrating hepatic macrophages, especially those expressing Gpnmb, during the repair process in injured livers in a mouse model of acute liver injury. [from: Kumagai K, Tabu K, Sasaki F, Takami Y, Morinaga Y, Mawatari S, et al. (2015) Glycoprotein Nonmetastatic Melanoma B (Gpnmb)-Positive Macrophages Contribute to the Balance between Fibrosis and Fibrolysis during the Repair of Acute Liver Injury in Mice. PLoS ONE 10(11): e0143413. https://doi.org/10.1371/journal.pone.0143413] |
| Host | RAB |
| Species specificity | HU |
| Figure 1 | 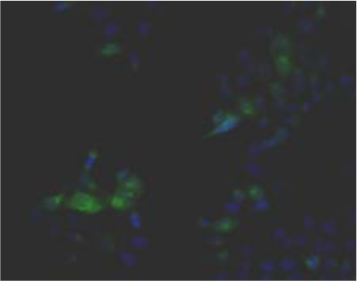 |
| Immunohistochemistry. Hela cells ICAFectin441-transfected with a plasmid encoding human GPNMB were fixed, permeabilized and stained with rabbit anti-human GPNMB polyclonal IgG antibody (diluted 1/100) and Alexa488 goat anti rabbit IgG secondary antibody. Nuclei were counterstained with DAPI. |
|
| Figure 2 | 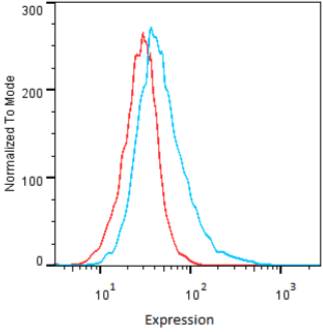 |
| Flow Cytometry. HeLa cells untransfected (red) and ICAFectin441-transfected with a plasmid encoding human GPNMB (blue) were stained with rabbit anti-human GPNMB polyclonal IgG antibody (diluted 1/100) and R-PE goat anti rabbit IgG secondary antibody. |
|
| Figure 3 | 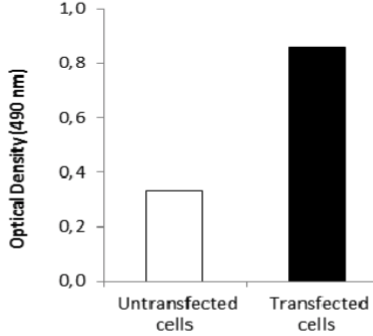 |
| ELISA. Lysates of HeLa cells untransfected (red) and ICAFectin441-transfected with a plasmid encoding human GPNMB were coated in 96 wells plate. Wells were stained with anti-human GPNMB polyclonal IgG and HRP goat anti rabbit IgG secondary antibody. |
|
| Product name | Anti Human/Rat Transmembrane Glycoprotein NMB (GPNMB) pAb (Rabbit, Purified Ig) |
| Cat No | CAC-ICA-TG1-RBP2 |
| Description | Originally described in melanoma cells, glycoprotein nonmetastatic melanoma B (Gpnmb), also known as osteoactivin and dendritic cell-associated heparin sulfate proteoglycan-dependent integrin ligand, is a heavily N-glycosylated type I transmembrane domain protein with a short cytoplasmic domain containing an endosomal sorting motif. Gpnmb is expressed in numerous cell types, including dendritic cells, macrophages, retinal pigment epithelial cells, osteoblasts and osteoclasts. Recent studies have demonstrated that Gpnmb expressed in macrophages functions as a feedback regulator of proinflammatory responses, and that binding of Gpnmb on antigen-presenting cells to syndecan-4 on activated T cells inhibits T-cell activation. Thus, Gpnmb expressed on antigen-presenting cells may negatively regulate inflammation. Additionally, Gpnmb is involved in osteoblast maturation, and is also associated with motor neuron survival. We previously isolated the gene encoding Gpnmb and demonstrated that it was differentially expressed in the livers of rats fed a choline-deficient, L-amino acid-defined diet. We also found that transgenic Gpnmb expression attenuates the development of hepatic fibrosis. Furthermore, a recent report has demonstrated that Gpnmb is expressed in macrophages infiltrating into injured liver tissues. These findings suggest a pathophysiologic role for Gpnmb in various disorders, including liver injury. However, the specific nature of Gpnmb involvement in liver injury remains unknown. In this study, we focused on the role of infiltrating hepatic macrophages, especially those expressing Gpnmb, during the repair process in injured livers in a mouse model of acute liver injury. [from: Kumagai K, Tabu K, Sasaki F, Takami Y, Morinaga Y, Mawatari S, et al. (2015) Glycoprotein Nonmetastatic Melanoma B (Gpnmb)-Positive Macrophages Contribute to the Balance between Fibrosis and Fibrolysis during the Repair of Acute Liver Injury in Mice. PLoS ONE 10(11): e0143413. https://doi.org/10.1371/journal.pone.0143413] |
| Host | RAB |
| Species specificity | HU RT |
| Figure 1 | 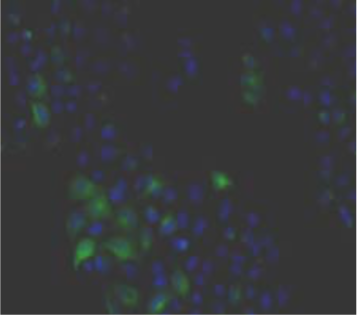 |
| Immunohistochemistry. Hela cells ICAFectin441-transfected with a plasmid encoding human GPNMB were fixed, permeabilized and stained with rabbit anti-human GPNMB polyclonal IgG antibody (diluted 1/100) and Alexa488 goat anti rabbit IgG secondary antibody. Nuclei were counterstained with DAPI. |
|
| Figure 2 | 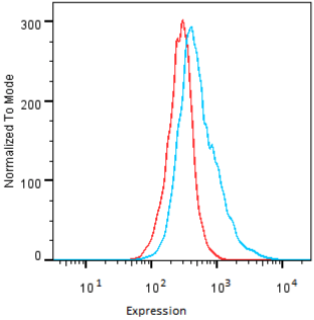 |
| Flow Cytometry. HeLa cells untransfected (red) and ICAFectin441-transfected with a plasmid encoding human GPNMB (blue) were stained with rabbit anti-human GPNMB polyclonal IgG antibody (diluted 1/100) and R-PE goat anti rabbit IgG secondary antibody. |
|
| Figure 3 | 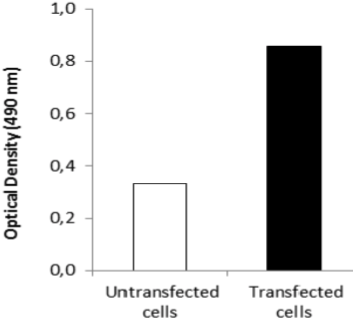 |
| ELISA. Lysates of HeLa cells untransfected (red) and ICAFectin441-transfected with a plasmid encoding human GPNMB were coated in 96 wells plate. Wells were stained with anti-human GPNMB polyclonal IgG and HRP goat anti rabbit IgG secondary antibody. |
|
| Product name | Anti Human/Rat/Mouse Transmembrane Glycoprotein NMB (GPNMB) pAb (Rabbit, Purified Ig) |
| Cat No | CAC-ICA-TG1-RBP3 |
| Description | Originally described in melanoma cells, glycoprotein nonmetastatic melanoma B (Gpnmb), also known as osteoactivin and dendritic cell-associated heparin sulfate proteoglycan-dependent integrin ligand, is a heavily N-glycosylated type I transmembrane domain protein with a short cytoplasmic domain containing an endosomal sorting motif. Gpnmb is expressed in numerous cell types, including dendritic cells, macrophages, retinal pigment epithelial cells, osteoblasts and osteoclasts. Recent studies have demonstrated that Gpnmb expressed in macrophages functions as a feedback regulator of proinflammatory responses, and that binding of Gpnmb on antigen-presenting cells to syndecan-4 on activated T cells inhibits T-cell activation. Thus, Gpnmb expressed on antigen-presenting cells may negatively regulate inflammation. Additionally, Gpnmb is involved in osteoblast maturation, and is also associated with motor neuron survival. We previously isolated the gene encoding Gpnmb and demonstrated that it was differentially expressed in the livers of rats fed a choline-deficient, L-amino acid-defined diet. We also found that transgenic Gpnmb expression attenuates the development of hepatic fibrosis. Furthermore, a recent report has demonstrated that Gpnmb is expressed in macrophages infiltrating into injured liver tissues. These findings suggest a pathophysiologic role for Gpnmb in various disorders, including liver injury. However, the specific nature of Gpnmb involvement in liver injury remains unknown. In this study, we focused on the role of infiltrating hepatic macrophages, especially those expressing Gpnmb, during the repair process in injured livers in a mouse model of acute liver injury. [from: Kumagai K, Tabu K, Sasaki F, Takami Y, Morinaga Y, Mawatari S, et al. (2015) Glycoprotein Nonmetastatic Melanoma B (Gpnmb)-Positive Macrophages Contribute to the Balance between Fibrosis and Fibrolysis during the Repair of Acute Liver Injury in Mice. PLoS ONE 10(11): e0143413. https://doi.org/10.1371/journal.pone.0143413] |
| Host | RAB |
| Species specificity | HU MS RT |
| Figure 1 | 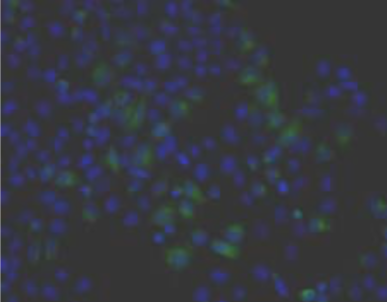 |
| Immunohistochemistry. Hela cells ICAFectin441-transfected with a plasmid encoding human GPNMB were fixed, permeabilized and stained with rabbit anti-human GPNMB polyclonal IgG antibody (diluted 1/100) and Alexa488 goat anti rabbit IgG secondary antibody. Nuclei were counterstained with DAPI. |
|
| Figure 2 | 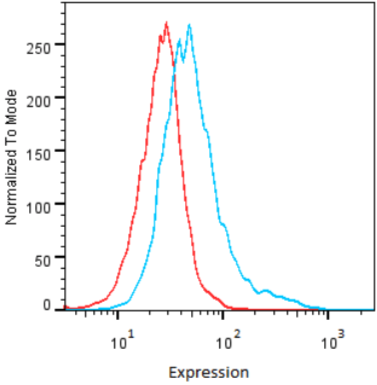 |
| Flow Cytometry. HeLa cells untransfected (red) and ICAFectin441-transfected with a plasmid encoding human GPNMB (blue) were stained with rabbit anti-human GPNMB polyclonal IgG antibody (diluted 1/100) and R-PE goat anti rabbit IgG secondary antibody. |
|
| Figure 3 | 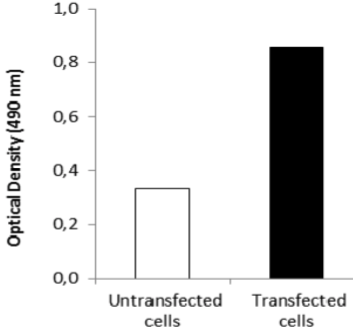 |
| ELISA. Lysates of HeLa cells untransfected (red) and ICAFectin441-transfected with a plasmid encoding human GPNMB were coated in 96 wells plate. Wells were stained with anti-human GPNMB polyclonal IgG and HRP goat anti rabbit IgG secondary antibody. |
|
