 CAC Antibody Collection
CAC Antibody Collection
The antibodies on this page are part of Cosmo Bio's exclusive CAC Collection. For many many thousands of other antibodies from many different makers, use our Search the Store function and our Explore Products drop down menu.
Chaperones
Molecular chaperones are diverse families of multidomain proteins that have evolved to assist nascent proteins to reach their native fold, protect subunits from heat shock during the assembly of complexes, prevent protein aggregation or mediate targeted unfolding and disassembly. Their increased expression in response to stress is a key factor in the health of the cell and longevity of an organism. Unlike enzymes with their precise and finely tuned active sites, chaperones are heavy-duty molecular machines that operate on a wide range of substrates. The structural basis of their mechanism of action is being unraveled (in particular for the heat shock proteins HSP60, HSP70, HSP90 and HSP100) and typically involves massive displacements of 20–30 kDa domains over distances of 20–50 Å and rotations of up to 100°. [from: Saibil, H.(2013) Chaperone machines for protein folding, unfolding and disaggregation. Nature Reviews Molecular Cell Biology. 14:630-642.]| Product name | Anti Alpha-Crystallin B Chain, p19S pAb (Rabbit, Affinity Purified) |
| Cat No | CAC-ACC-PA004 |
| Description | Lens proteins consist almost entirely of crystallins (about 95%). Crystallins are also found in vertebrate skeletal muscle tissue. In the lens, their structural function is to assist in maintaining the proper refractive index of the lens. The mammalian lens contains 3 major classes of crystallins: alpha, beta, and gamma. Alpha-crystallin is the largest of the crystallins and is composed of 2 primary gene products, alpha-A and alpha-B. There are at least 5 different proteins comprising the beta-crystallins. The gamma-crystallins are monomeric, but there are at least 5 gamma crystallins identified in bovine and rat lens. Alpha-Crystallin comprises 40% of total lens protein composition. In addition to maintaining proper refractive index, it also functions in a chaperone like manner by preventing the formation of aggregates possibly leading to cataract formation. It is believed that the phosphorylated states of alpha-crystallin occur in response to cellular stress and may serve a structural control function and play a role in protein maintenance. Alpha-B crystallin has been linked to Alexander, Alzheimer’s and Parkinson’s diseases where it accumulates in brain cells. References: 1) Ito H, Okamoto K, Nakayama H, Isobe T, Kato K. (1997) Phosphorylation of B-crystallin in response to various types of stress. J Biol Chem. 272:29934-29941. 2) Kato K, Ito H, Kamei K, Inaguma Y, Iwamoto I, Saga S. (1998) Phosphorylation of B-crystallin in mitotic cells and identification of enzymatic activities responsible for phosphorylation. J Biol Chem. 273:28346-28354. 3) Ito H, Iida K, Kamei K, Iwamoto I, Inaguma Y, Kato K. (1999) B-crystallin in rat lens is phosphorylated at an early postnatal age. FEBS Lett. 446:269-272. |
| Host | RAB |
| Species specificity | HU MS RT BOV |
| Product name | Anti Alpha-Crystallin B Chain, p45S pAb (Rabbit, Affinity Purified) |
| Cat No | CAC-ACC-PA005 |
| Description | Lens proteins consist almost entirely of crystallins (about 95%). Crystallins are also found in vertebrate skeletal muscle tissue. In the lens, their structural function is to assist in maintaining the proper refractive index of the lens. The mammalian lens contains 3 major classes of crystallins: alpha, beta, and gamma. Alpha-crystallin is the largest of the crystallins and is composed of 2 primary gene products, alpha-A and alpha-B. There are at least 5 different proteins comprising the beta-crystallins. The gamma-crystallins are monomeric, but there are at least 5 gamma crystallins identified in bovine and rat lens. Alpha-Crystallin comprises 40% of total lens protein composition. In addition to maintaining proper refractive index, it also functions in a chaperone like manner by preventing the formation of aggregates possibly leading to cataract formation. It is believed that the phosphorylated states of alpha-crystallin occur in response to cellular stress and may serve a structural control function and play a role in protein maintenance. Alpha-B crystallin has been linked to Alexander, Alzheimer’s and Parkinson’s diseases where it accumulates in brain cells. References: 1) Ito H, Okamoto K, Nakayama H, Isobe T, Kato K. (1997) Phosphorylation of B-crystallin in response to various types of stress. J Biol Chem. 272:29934-29941. 2) Kato K, Ito H, Kamei K, Inaguma Y, Iwamoto I, Saga S. (1998) Phosphorylation of B-crystallin in mitotic cells and identification of enzymatic activities responsible for phosphorylation. J Biol Chem. 273:28346-28354. 3) Ito H, Iida K, Kamei K, Iwamoto I, Inaguma Y, Kato K. (1999) B-crystallin in rat lens is phosphorylated at an early postnatal age. FEBS Lett. 446:269-272. |
| Host | RAB |
| Species specificity | HU MS RT BOV |
| Figure 1 | 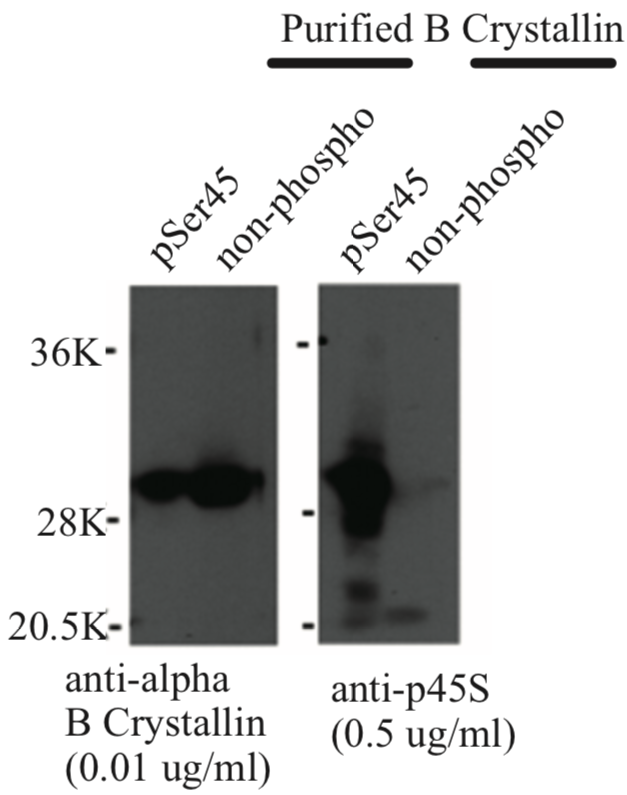 |
| Immunoblot analysis of Anti alpha-B crystallin p45S antibody. | |
| Product name | Anti Alpha-Crystallin B Chain, p59S pAb (Rabbit, Affinity Purified) |
| Cat No | CAC-ACC-PA006 |
| Description | Lens proteins consist almost entirely of crystallins (about 95%). Crystallins are also found in vertebrate skeletal muscle tissue. In the lens, their structural function is to assist in maintaining the proper refractive index of the lens. The mammalian lens contains 3 major classes of crystallins: alpha, beta, and gamma. Alpha-crystallin is the largest of the crystallins and is composed of 2 primary gene products, alpha-A and alpha-B. There are at least 5 different proteins comprising the beta-crystallins. The gamma-crystallins are monomeric, but there are at least 5 gamma crystallins identified in bovine and rat lens. Alpha-Crystallin comprises 40% of total lens protein composition. In addition to maintaining proper refractive index, it also functions in a chaperone like manner by preventing the formation of aggregates possibly leading to cataract formation. It is believed that the phosphorylated states of alpha-crystallin occur in response to cellular stress and may serve a structural control function and play a role in protein maintenance. Alpha-B crystallin has been linked to Alexander, Alzheimer’s and Parkinson’s diseases where it accumulates in brain cells. References: 1) Ito H, Okamoto K, Nakayama H, Isobe T, Kato K. (1997) Phosphorylation of B-crystallin in response to various types of stress. J Biol Chem. 272:29934-29941. 2) Kato K, Ito H, Kamei K, Inaguma Y, Iwamoto I, Saga S. (1998) Phosphorylation of B-crystallin in mitotic cells and identification of enzymatic activities responsible for phosphorylation. J Biol Chem. 273:28346-28354. 3) Ito H, Iida K, Kamei K, Iwamoto I, Inaguma Y, Kato K. (1999) B-crystallin in rat lens is phosphorylated at an early postnatal age. FEBS Lett. 446:269-272. |
| Host | RAB |
| Species specificity | HU RT BOV |
| Figure 1 | 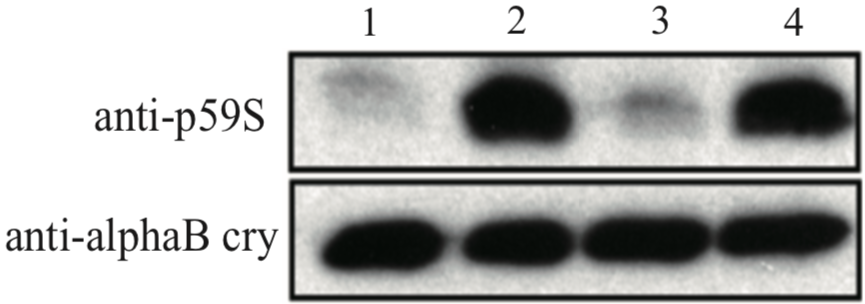 |
| Immunoblot analysis of anti alpha-B crystallin p59S antibody. U373MG cell lysates: 1: control 2: 4mM H2O2 for 90min 3: 4mM H2O2 + 10uM SB203580 for 90min 4: 4mM H2O2 + 30uM SB203580 for 90min |
|
