 CAC Antibody Collection
CAC Antibody Collection
The antibodies on this page are part of Cosmo Bio's exclusive CAC Collection. For many many thousands of other antibodies from many different makers, use our Search the Store function and our Explore Products drop down menu.
Mitochondria-related
The mitochondrion (plural mitochondria) is a double-membrane-bound organelle found in most eukaryotic organisms. Some cells in some multicellular organisms may, however, lack them (for example, mature mammalian red blood cells). A number of unicellular organisms, such as microsporidia, parabasalids, and diplomonads, have also reduced or transformed their mitochondria into other structures. To date, only one eukaryote, Monocercomonoides, is known to have completely lost its mitochondria. The word mitochondrion comes from the Greek μίτος, mitos, "thread", and χονδρίον, chondrion, "granule" or "grain-like". Mitochondria generate most of the cell's supply of adenosine triphosphate (ATP), used as a source of chemical energy. A mitochondrion is thus termed the powerhouse of the cell. Mitochondria are commonly between 0.75 and 3 μm in diameter but vary considerably in size and structure. Unless specifically stained, they are not visible. In addition to supplying cellular energy, mitochondria are involved in other tasks, such as signaling, cellular differentiation, and cell death, as well as maintaining control of the cell cycle and cell growth. Mitochondrial biogenesis is in turn temporally coordinated with these cellular processes. Mitochondria have been implicated in several human diseases, including mitochondrial disorders, cardiac dysfunction, heart failure and autism. The number of mitochondria in a cell can vary widely by organism, tissue, and cell type. For instance, red blood cells have no mitochondria, whereas liver cells can have more than 2000. The organelle is composed of compartments that carry out specialized functions. These compartments or regions include the outer membrane, the intermembrane space, the inner membrane, and the cristae and matrix. Although most of a cell's DNA is contained in the cell nucleus, the mitochondrion has its own independent genome that shows substantial similarity to bacterial genomes. Mitochondrial proteins (proteins transcribed from mitochondrial DNA) vary depending on the tissue and the species. In humans, 615 distinct types of protein have been identified from cardiac mitochondria, whereas in rats, 940 proteins have been reported. The mitochondrial proteome is thought to be dynamically regulated. [from: Wikipedia contributors. (2019, May 31). Mitochondrion. In Wikipedia, The Free Encyclopedia. Retrieved 18:12, June 4, 2019, from https://en.wikipedia.org/w/index.php?title=Mitochondrion&oldid=899605686]
| Product name (click for order info) | Cat No (click for datasheet) |
Host | Species specificity |
| Anti Prohibitin-1 (PHB1) mAb (Clone 1G5C1) | CAC-CE-049A | RT | HU MS RT MKY |
| Anti Prohibitin-2 (PHB2) mAb (Clone 7F8B3) | CAC-CE-050A | RT | HU MS RT MKY |
| Anti Translocator Protein (TSPO) pAb (Mouse, Antiserum) | CAC-ICA-TG5-MSP1 | MS | MS |
| Anti Translocator Protein (TSPO) pAb (Rabbit, Antiserum) | CAC-ICA-TG5-RBP1 | RAB | MS |
| Product name | Anti Prohibitin1 (PHB1) mAb (Clone 1G5C1) |
| Cat No | CAC-CE-049A |
| Description | Prohibitin, also known as PHB, is a protein that in humans is encoded by the PHB gene. The Phb gene has also been described in animals, fungi, plants, and unicellular eukaryotes. Prohibitins are divided in two classes, termed Type-I and Type-II prohibitins, based on their similarity to yeast PHB1 and PHB2, respectively. Each organism has at least one copy of each type of prohibitin gene. Prohibitins are evolutionarily conserved genes that are ubiquitously expressed. The human prohibitin gene, located on the BRCA1 chromosome region 17q21, was originally thought to be a negative regulator of cell proliferation and a tumor suppressor. This anti-proliferative activity was later attributed to the 3' UTR of the PHB gene, and not to the actual protein. Mutations in human PHB have been linked to sporadic breast cancer. However, over-expression of PHB has been associated with a reduction in the androgen receptor activity and a reduction in PSA gene expression resulting in a decrease of androgen-dependent growth of cancerous prostate cells. Prohibitin is expressed as two transcripts with varying lengths of 3' untranslated region. The longer transcript is present at higher levels in proliferating tissues and cells, suggesting that this longer 3' untranslated region may function as a trans-acting regulatory RNA. Prohibitins are assembled into a ring-like structure with 16–20 alternating Phb1 and Phb2 subunits in the inner mitochondrial membrane. The precise molecular function of the PHB complex is not clear, but a role as chaperone for respiration chain proteins or as a general structuring scaffold required for optimal mitochondrial morphology and function are suspected. Recently, prohibitins have been demonstrated to be positive, rather than negative, regulators of cell proliferation in both plants and mice. Both human prohibitins have also been suggested to be localized in the nucleus and modulate transcriptional activity by interacting with various transcription factors, including nuclear receptors, either directly or indirectly. However, little evidence for nuclear targeting and transcription factor-binding of prohibitins has been found in other organisms (yeast, plants, C. elegans, etc.), indicating that this may be a specific function in mammalian cells. [from: Wikipedia contributors. (2019, February 20). Prohibitin. In Wikipedia, The Free Encyclopedia. Retrieved 18:24, June 4, 2019, from https://en.wikipedia.org/w/index.php?title=Prohibitin&oldid=884240489] References: 1) Schneider et al. (2010) Prohibitin1 acts as a neural crest specifier in Xenopus development by repressing the transcription factor E2F1. Development. 137:4073-4081. |
| Host | RT |
| Species specificity | HU MS RT MKY |
| Figure 1 | 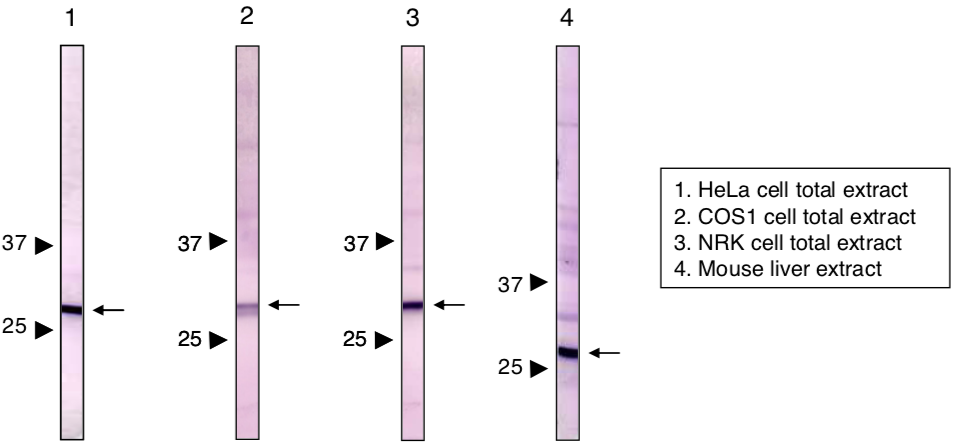 |
| Immunoblot analysis of Prohibitin1 (PHB1) antibody (1G5C1). | |
| Product name | Anti Prohibitin2 (PHB2) mAb (Clone 7F8B3) |
| Cat No | CAC-CE-050A |
| Description | Prohibitin, also known as PHB, is a protein that in humans is encoded by the PHB gene. The Phb gene has also been described in animals, fungi, plants, and unicellular eukaryotes. Prohibitins are divided in two classes, termed Type-I and Type-II prohibitins, based on their similarity to yeast PHB1 and PHB2, respectively. Each organism has at least one copy of each type of prohibitin gene. Prohibitins are evolutionarily conserved genes that are ubiquitously expressed. The human prohibitin gene, located on the BRCA1 chromosome region 17q21, was originally thought to be a negative regulator of cell proliferation and a tumor suppressor. This anti-proliferative activity was later attributed to the 3' UTR of the PHB gene, and not to the actual protein. Mutations in human PHB have been linked to sporadic breast cancer. However, over-expression of PHB has been associated with a reduction in the androgen receptor activity and a reduction in PSA gene expression resulting in a decrease of androgen-dependent growth of cancerous prostate cells. Prohibitin is expressed as two transcripts with varying lengths of 3' untranslated region. The longer transcript is present at higher levels in proliferating tissues and cells, suggesting that this longer 3' untranslated region may function as a trans-acting regulatory RNA. Prohibitins are assembled into a ring-like structure with 16–20 alternating Phb1 and Phb2 subunits in the inner mitochondrial membrane. The precise molecular function of the PHB complex is not clear, but a role as chaperone for respiration chain proteins or as a general structuring scaffold required for optimal mitochondrial morphology and function are suspected. Recently, prohibitins have been demonstrated to be positive, rather than negative, regulators of cell proliferation in both plants and mice. Both human prohibitins have also been suggested to be localized in the nucleus and modulate transcriptional activity by interacting with various transcription factors, including nuclear receptors, either directly or indirectly. However, little evidence for nuclear targeting and transcription factor-binding of prohibitins has been found in other organisms (yeast, plants, C. elegans, etc.), indicating that this may be a specific function in mammalian cells. [from: Wikipedia contributors. (2019, February 20). Prohibitin. In Wikipedia, The Free Encyclopedia. Retrieved 18:24, June 4, 2019, from https://en.wikipedia.org/w/index.php?title=Prohibitin&oldid=884240489] References: 1) Sun et al. (2011) CaMK IV phosphorylates prohibitin 2 and regulates prohibitin 2-mediated repression of MEF2 transcription. Cell Signal. 23:1686-1690. |
| Host | RT |
| Species specificity | HU MS RT MKY |
| Figure 1 | 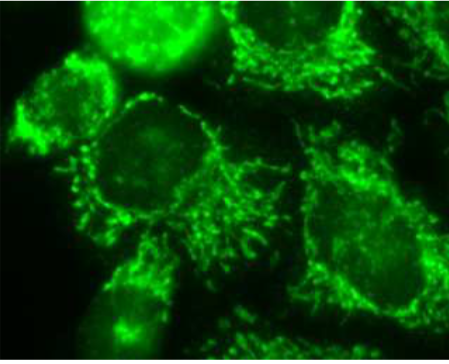 |
| Immunocytochemistry / Immunofluorescence analysis of Prohibitin2 antibody (7F8E3) in HeLa cells. | |
| Figure 2 | 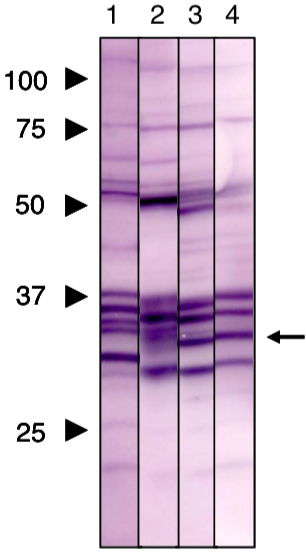 |
| Immunoblot analysis of Prohibitin2 antibody (7F8E3). 1) HeLa cell extract 2) COS1 cell extract 3) NRK cell extract 4) Mouse liver extract |
|
| Product name | Anti Translocator Protein (TSPO) pAb (Mouse, Antiserum) |
| Cat No | CAC-ICA-TG5-MSP1 |
| Description | Translocator protein (TSPO) is an 18 kDa protein mainly found on the outer mitochondrial membrane. It was first described as peripheral benzodiazepine receptor (PBR), a secondary binding site for diazepam, but subsequent research has found the receptor to be expressed throughout the body and brain. In humans, the translocator protein is encoded by the TSPO gene. It belongs to family of tryptophan-rich sensory proteins. Regarding intramitochondrial cholesterol transport, TSPO has been proposed to interact with StAR (steroidogenic acute regulatory protein) to transport cholesterol into mitochondria, though evidence is mixed. In animals, TSPO (PBR) is a mitochondrial protein usually located in the outer mitochondrial membrane and characterized by its ability to bind a variety of benzodiazepine-like drugs, as well as to dicarboxylic tetrapyrrole intermediates of the haem biosynthetic pathway. TSPO has many proposed functions depending on the tissue. The most studied of these include roles in the immune response, steroid synthesis and apoptosis. [from: Wikipedia contributors. (2019, April 29). Translocator protein. In Wikipedia, The Free Encyclopedia. Retrieved 18:37, June 4, 2019, from https://en.wikipedia.org/w/index.php?title=Translocator_protein&oldid=894679824] |
| Host | MS |
| Species specificity | MS |
| Figure 1 | 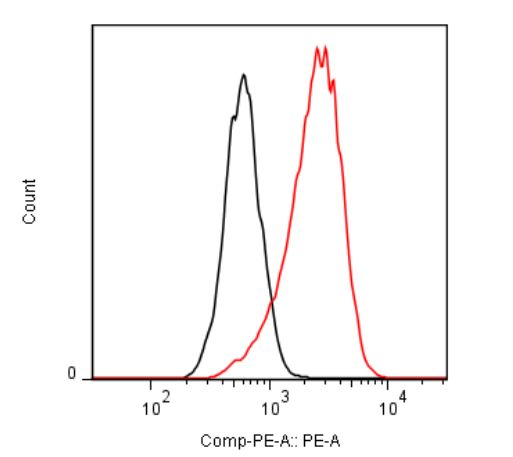 |
| Flow cytometric analysis. Hela cells were untransfected (black) or ICAFectin®441-transfected with a plasmid encoding mouse TSPO (red). Hela cells were fixed 24h post transfection, permeabilized and stained with mouse anti-mouse TSPO polyclonal antibodies diluted 1/500 and R-PE goat anti mouse IgG secondary antibody diluted 1/200. | |
| Product name | Anti Translocator Protein (TSPO) pAb (Rabbit, Antiserum) |
| Cat No | CAC-ICA-TG5-RBP1 |
| Description | Translocator protein (TSPO) is an 18 kDa protein mainly found on the outer mitochondrial membrane. It was first described as peripheral benzodiazepine receptor (PBR), a secondary binding site for diazepam, but subsequent research has found the receptor to be expressed throughout the body and brain. In humans, the translocator protein is encoded by the TSPO gene. It belongs to family of tryptophan-rich sensory proteins. Regarding intramitochondrial cholesterol transport, TSPO has been proposed to interact with StAR (steroidogenic acute regulatory protein) to transport cholesterol into mitochondria, though evidence is mixed. In animals, TSPO (PBR) is a mitochondrial protein usually located in the outer mitochondrial membrane and characterized by its ability to bind a variety of benzodiazepine-like drugs, as well as to dicarboxylic tetrapyrrole intermediates of the haem biosynthetic pathway. TSPO has many proposed functions depending on the tissue. The most studied of these include roles in the immune response, steroid synthesis and apoptosis. [from: Wikipedia contributors. (2019, April 29). Translocator protein. In Wikipedia, The Free Encyclopedia. Retrieved 18:37, June 4, 2019, from https://en.wikipedia.org/w/index.php?title=Translocator_protein&oldid=894679824] |
| Host | RAB |
| Species specificity | MS |
| Figure 1 | 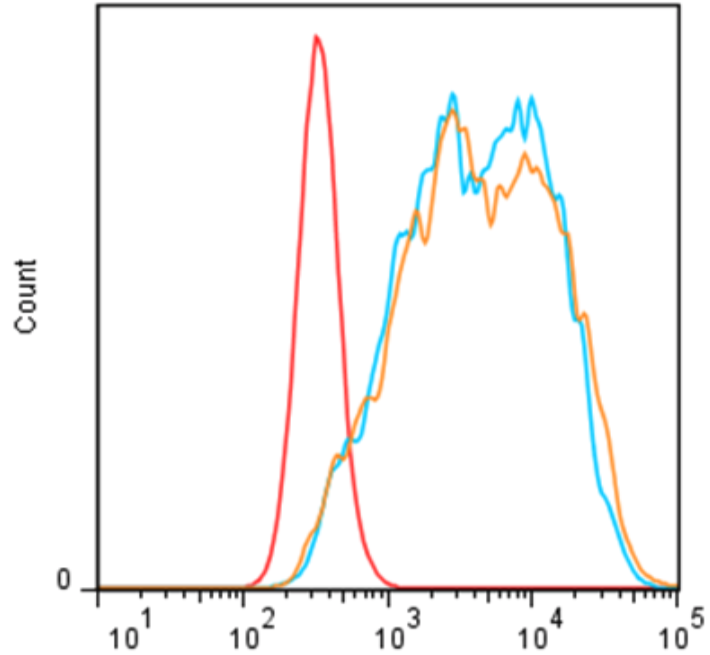 |
| Flow cytometric analysis. Hela cells were untransfected (black) or ICAFectin®441-transfected with a plasmid encoding mouse TSPO (red). Hela cells were fixed 24h post transfection, permeabilized and stained with rabbit anti-mouse TSPO polyclonal antibody diluted 1/100 (orange) and 1/200 (blue) and R-PE goat anti rabbit IgG secondary antibody diluted 1/200./td> | |
| Figure 2 | 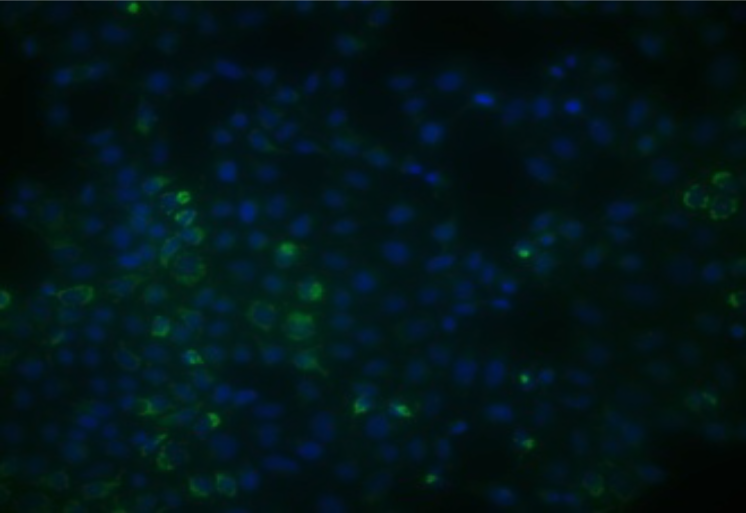 |
| Immunohistochemical analysis. Hela cells ICAFectin®441-transfected with a plasmid encoding mouse TSPO were fixed 24h post transfection, permeabilized and stained with rabbit anti-mouse TSPO polyclonal antibody diluted 1/20 and Alexa488 goat anti rabbit IgG secondary antibody diluted 1/1000. Nuclei were counterstained with DAPI in blue. | |
