- CAC Antibody Collection Index Page
Antibody Group
Advanced glycation end products (AGEs)
Autophagy and apoptosis
Bacteria-related
Calcium-binding proteins
Cancer
CD44 for enriching cancer stem cells
Tumor markers
Tumor inhibitors
Chaperones
Cytoskeleton
DNA damage
UV-induced DNA lesions
8-Nitroguanosine for oxidative stress research
Nucleotide excision repair factors
Epigenetics and chromatin
Histone H3 variants
Post-translationally-modified histone H3
Chromatin structure modifiers
Drosophila chromatin
Epitope tags
Exosomes
Extracellular matrix
Glycosaminoglycans (GAGs)
Proteoglycans
Matrix and basement membrane
Cell adhesion and hemidesmosome-related
Bone and cartilage-related
Wound repair-related
Hedgehog pathway
Hormones
Immunology
Fish CD4 and CD8α
Allergic disease-related
Adaptive and innate immunity
Macrophages
Inflammatory cytokines
Viral recognition pathways
Vpr for HIV research
Insulin-like growth factor-related
Mitochondria-related
Neurobiology
Neurodegenerative disease markers
Muscarinic acetylcholine receptors
Miscellaneous
Nuclear import and export
Oxidative stress
Plant-related
Plant hormones
Plant autophagy and apoptosis
Plant stress response
Plant stress response
Proteasomes
Puromycin-specific
Reproductive biology
Small molecules
Stem cells
Novel iPS/ES markers
Pluripotency-associated
Sumoylation pathway
TGF-beta pathway
TGF-beta LAP-d
TGF-beta signaling
Transcription factors
Transporters
Tyrosine phosphatases
Ubiquitin-Proteasome Related
 CAC Antibody Collection
CAC Antibody Collection
The antibodies on this page are part of Cosmo Bio's exclusive CAC Collection. For many many thousands of other antibodies from many different makers, use our Search the Store function and our Explore Products drop down menu.
Advanced glycation end products (AGEs)
A heterogeneous group of molecules collectively called advanced glycation end products (AGEs), are produced in the classical Maillard reaction, discovered at the beginning of the 20th century (Maillard, 1912). More than three decades ago, Monnier and Cerami proposed a Maillard theory of aging postulating that slow and continuous accumulation of AGEs was a causal factor in aging (Bjorksten, 1968; Monnier, 1989; Monnier et al., 1988; Sell and Monnier, 1989). Furthermore, they proposed that the protracted buildup of these compounds may alter the structure and function of proteins, thus affecting several of the hallmarks of aging (Gugliucci and Menini, 2017; Lopez-Otin et al., 2013). This process may also contribute to the pathology of metabolic diseases, such as diabetes and atherosclerosis, as well as oxidative stress and inflammation associated with neurodegenerative diseases of aging. Support for this hypothesis includes an age-dependent increase in browning (Maillard reaction), fluorescence, cross-linking, insolubility, and accrual of AGEs in collagens and lens crystallins (Monnier et al., 1984). Despite this accumulating evidence, debate continues over whether AGEs are causal or just a consequence of aging and age-related diseases (Gugliucci, 2017).
The link between age-related diseases and AGEs has been difficult to unravel for several reasons: the variety of sources for AGEs, the gradual buildup of AGEs, which can take decades to detect in humans, lack of accessible and sensitive methods to quantify specific AGEs, a growing number of targets of AGEs, and a lack of models that recapitulate the pathologies resulting from the accumulation of AGEs. These factors have complicated efforts to model causation by connecting an AGE to one specific target and a specific disease relevant to aging. [from: Chaudhuri J., Bains Y., Guha S., Kahn A., Hall D., Bose N., Gugliucci A., Kapahi P. The Role of Advanced Glycation End Products in Aging and Metabolic Diseases: Bridging Association and Causality (2018) Cell Metabolism 28(3):337-352]
| Product name (click for order info) | Cat No (click for datasheet) |
Host | Species specificity |
| Anti N(EPSILON)-(Carboxymethyl) lysine (CML) mAb (Clone 2G11) | CAC-AGE-M01 | MS | - |
| Anti N(OMEGA)-(Carboxymethyl) arginine (CMA) mAb (Clone 3F5) | CAC-AGE-M04 | MS | - |
| Anti N(EPSILON)-(Carboxyethyl) lysine (CEL) mAb (Clone CEL-SP) | CAC-AGE-M02 | MS | - |
| Anti GA-Pyridine mAb (Clone 2A2) | CAC-AGE-M03 | MS | - |
| Product name | Anti N(Epsilon)-(Carboxymethyl) Lysine (CML) mAb (Clone 2G11) |
| Cat No | CAC-AGE-M01 |
| Description | Nε-(carboxymethyl) lysine (CML) is a major antigenic AGE structure. Recent studies demonstrate that CML is generated by the oxidative cleavage of Amadori products by hydroxyl radicals, peroxynitrite and hypochlorous acid, suggesting CML to be an important marker of oxidative stress in vivo. CML is the most used marker for AGEs in food analysis. Source: Professor Nagai Ryuji, Tokai University Faculty of Agriculture Department of Biosciences Food Bioregulation Research Laboratory. References: Mera K., Nagai M., Brock JW., Fujiwara Y., Imai H., Murata T., Maruyama T., Baynes JW., Otagiri M., Nagai R. Glutaraldehyde is an Effective Cross-linker for Production of Antibodies Against Advanced Glycation End Products. J. Immunol. Methods 334 (1-2), 82-90 (2008) PMID: 18353354 |
| Host | Mouse |
| Species specificity | - |
| Figure 1 | 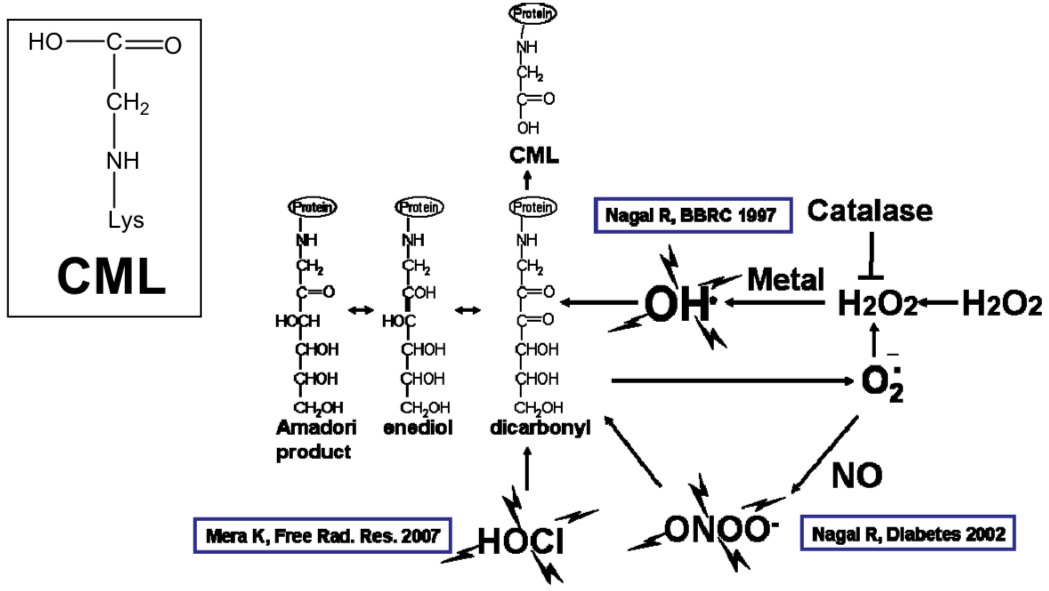 |
| CML production pathway. | |
| Figure 2 | 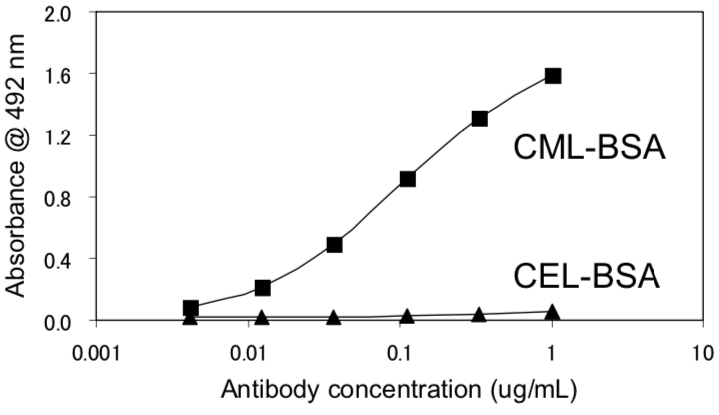 |
| Immunoreactivity of the CML(2G11) monoclonal antibody to CML-BSA and CEL-BSA. | |
| Product name | Anti N(Omega)-(Carboxymethyl) Arginine (CMA) mAb (Clone 3F5) |
| Cat No | CAC-AGE-M04 |
| Description | Nω-(carboxymethyl) arginine (CMA), a CML analogue, is an acid-labile AGE structure which was discovered in an enzymatic hydrolysate of glycated collagen. CMA is preferentially generated in glycated collagen. Source: Professor Nagai Ryuji, Tokai University Faculty of Agriculture Department of Biosciences Food Bioregulation Research Laboratory. References: 1) Iijima K, Murata M, Takahara H, Irie S, Fujimoto D. Identification of N(omega)-carboxymethylarginine as a novel acid-labileadvanced glycation end product in collagen. Biochem J. 347 Pt 1:23-27 (2000) PMID: 10727397 2) Mera K., Fujiwara Y., Otagiri M., Sakata N., Nagai R. Immunological Detection of Nε -carboxymethylarginine by Specific Antibody. Ann N Y Acad Sci. 1126, 155-157 (2008) PMID: 18079475 |
| Host | Mouse |
| Species specificity | - |
| Figure 1 | 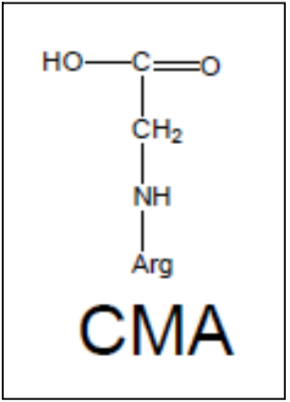 |
| Nω- (carboxymethyl) arginine (CMA) structure. | |
| Figure 2 | 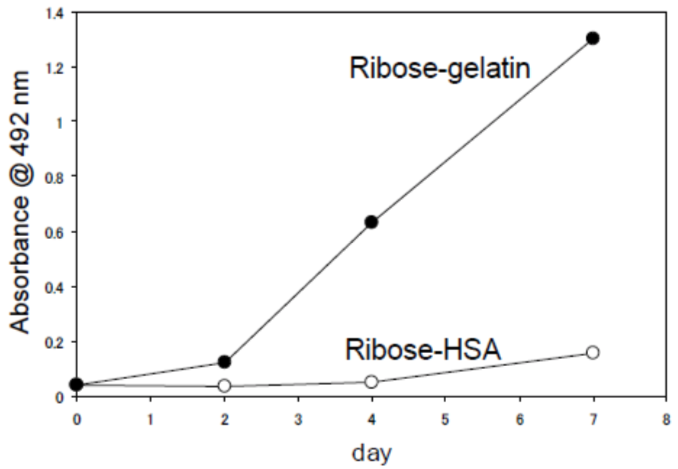 |
| Immunoreactivity of the CMA monoclonal antibody (3F5) to Ribose-gelatin and Ribose-HAS. | |
| Product name | Anti N(Epsilong)-(Carboxyethyl) Lysine (CEL) mAb (Clone CEL-SP) |
| Cat No | CAC-AGE-M02 |
| Description | Nε-(carboxyethyl) lysine (CEL) is generated from protein modification by methylglyoxal (MG). MG is enzymatically derived from the Embden-Meyerhof and polyol pathways, through the degradation of glyceraldehyde-3-phosphate (G3P) (Phillips and Thornalley, 1993). Mclellan et al. (McLellan et al., 1994) demonstrated that plasma MG concentration in insulin-dependent diabetic patients was 7-times higher than in healthy individuals. CEL accumulation increases with age in human lens proteins. Source: Professor Nagai Ryuji, Tokai University Faculty of Agriculture Department of Biosciences Food Bioregulation Research Laboratory. References: Nagai R., Fujiwara Y., Mera K., Yamagata K., Sakashita N., Takeya M. Immunochemical detection of Nε-(carboxyethyl)lysine using a specific antibody. J. Immunol. Methods 332, 112-120 (2008). PMID: 18242632 |
| Host | Mouse |
| Species specificity | - |
| Figure 1 | 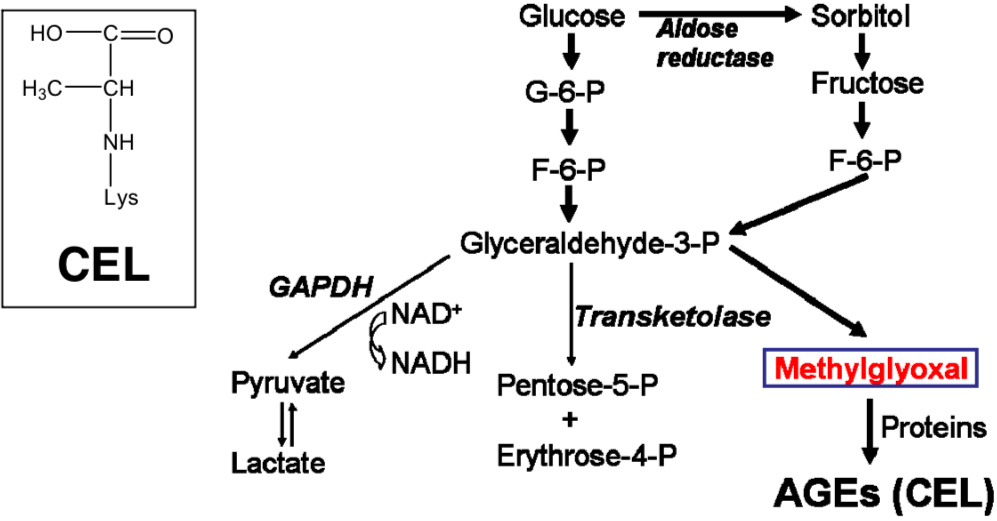 |
| CEL production pathway. | |
| Figure 2 | 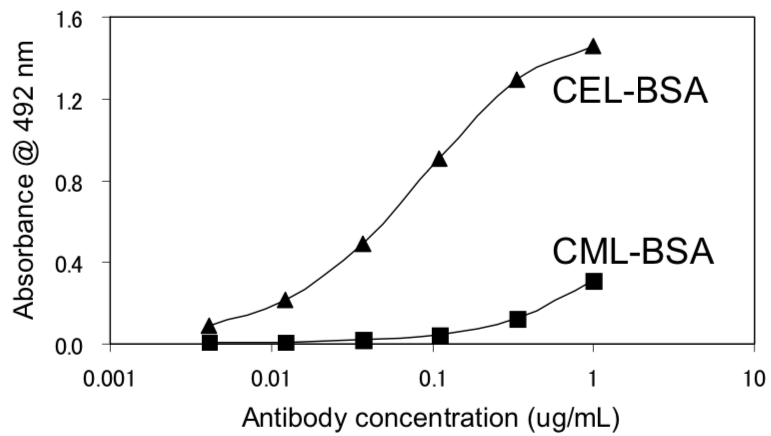 |
| Immunoreactivity of the CEL-SP monoclonal antibody to CEL-BSA and CML-BSA. | |
| Product name | Anti GA-Pyridine mAb (Clone 2A2) |
| Cat No | CAC-AGE-M03 |
| Description | Glycolaldehyde formed as a result of the myeloperoxidase-H2O2 (MPO) reaction can react with proteins to yield various AGEs. Recently, a novel GA-derived AGE, called GA-pyridine, has been described in foam cells and the extracellular matrix of human atherosclerotic fibrotic lesions, glomerular mesangium and Bruch’s membrane and choroid. Since GA-pyridine significantly accumulates in foamy macrophages of atherosclerotic plaques, it is thought that the modification of proteins with glycolaldehyde (GA) is involved in the development of arteriosclerosis. Source: Professor Nagai Ryuji, Tokai University Faculty of Agriculture Department of Biosciences Food Bioregulation Research Laboratory. References: 1) Nagai R., Hayashi CM., Xia L., Takeya M., Horiuchi S: Identification in human atherosclerotic lesions of GA-pyridine, a novel structure derived from glycolaldehyde-modified proteins. J Biol Chem. 277, 48905-48912 (2002). PMID: 12377783 2) Glenn JV., Mahaffy H., Wu K., Smith G., Nagai R., Simpson DAC., Boulton ME., Stitt AW. Advanced Glycation End Product (AGE) Accumulation on Bruch’s Membrane: Links to Age-Related RPE Dysfunction. Invest. Ophth. Vis. Sci. 50, 441-451 (2009). PMID: 18676633 |
| Host | Mouse |
| Species specificity | - |
| Figure 1 | 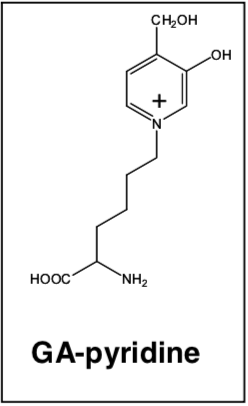 |
| GA-pyridine structure. | |
| Figure 2 | 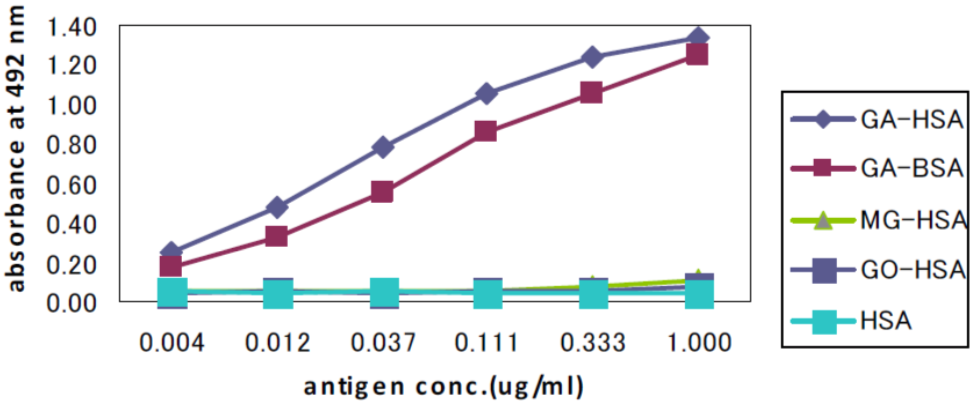 |
| Immunoreactivity of the GA-pyridine (2A2) monoclonal antibody to GA-HAS, GA-BSA, MG-HAS, GO-HSA and HAS. | |
