 CAC Antibody Collection
CAC Antibody Collection
The antibodies on this page are part of Cosmo Bio's exclusive CAC Collection. For many many thousands of other antibodies from many different makers, use our Search the Store function and our Explore Products drop down menu.
Neurodegenerative disease markers
Neurodegenerative diseases such as Alzheimer's disease and Parkinson's disease have been increasing rapidly and have become a serious social problem. In recent years, new causative genes have been discovered for amyotrophic lateral sclerosis (ALS) and other intractable neurological diseases opening new avenues for research on pathogenesis. It has been suggested that aggregation and accumulation of specific proteins cause neurotoxicity and the formation of lesions, but the onset and progression mechanisms are still unclear. Neuropathological diagnostic and experimental model biomarkers are needed for drug construction, drug discovery, and therapeutic development.
| Neurobiology: Muscarinic acetylcholine receptors | |||
| Product name (click for order info) | Cat No (click for datasheet) |
Host | Species specificity |
| Anti Muscarinic Acetylcholine Receptor M1(CHRM1) pAb (Rabbit, Antiserum) | CAC-YCU-PS-M1 | RAB | HU POR |
| Anti Muscarinic Acetylcholine Receptor M2 (CHRM2) pAb (Rabbit, Antiserum) | CAC-YCU-PS-M2 | RAB | HU POR |
| Anti Muscarinic Acetylcholine Receptor M3 (CHRM3) pAb (Rabbit, Antiserum) | CAC-YCU-PS-M3 | RAB | HU RT |
| Anti Muscarinic Acetylcholine Receptor M4 (CHRM4) pAb (Rabbit, Antiserum) | CAC-YCU-PS-M4 | RAB | HU RT |
| Neurobiology: Miscellaneous | |||
| Product name (click for order info) | Cat No (click for datasheet) |
Host | Species specificity |
| Anti Calcium/Calmodulin-Dependent Protein Kinase II, Isoform C (dCAMKII) mAb (Clone 18) | CAC-TNL-001-CAM | MS | Drosophila |
| Anti Schwann Cell/Peripheral Myelin mAb (Clone Schwann/2E) | CAC-GU01-M01AS-A | MS | HU MS RT |
| Product name | Anti 4R-Tau pAb (Rabbit, Antiserum) | |
| Cat No | CAC-TIP-4RT-P01 | |
| Description | Many neurodegenerative diseases such as Alzheimer's disease (AD) show abnormal lesions in which tau is fibrillated with a distribution that strongly correlates with clinical symptoms and disease progression. Tau is a microtubule-associated protein. In adult human brain, 6 tau isoforms are expressed, which are divided into two groups, 3-repeat (3R) and 4-repeat (4R) tau. Tau assembles into insoluble, filamentous, and hyperphosphorylated inclusions in several neurodegenerative diseases. Different tau isoforms accumulate in different neurodegenerative diseases: all 6 tau isoforms in AD, 3R tau isoforms in Pick’s disease, and 4R tau isoforms in cortico-basal degeneration (CBD) and progressive supranuclear palsy (PSP). Therefore, isoform-specific tau antibodies are useful tools for immunohistochemical and biochemical studies of tau species in diseased brains. Currently, a commercial antibody that recognizes 3R tau and 4R tau is widely used for biochemical and histologic analysis. However, tau deposited in AD is extensively deamidated at N279. Our anti-4R tau antibody specifically recognizes 4R tau isoforms regardless of deamidation and strongly stains tau in AD brains. References: 1) Hasegawa M, Watanabe S, Kondo H, et al, 3R and 4R tau isoforms in paired helical filaments in Alzheimer's disease. Acta Neuropathol. 2013 Nov 9 2) Dan A, Takahashi M, Masuda-Suzukake M, et al.Extensive deamidation at asparagine residue 279 accounts for weak immunoreactivity of tau with RD4 antibody in Alzheimer's disease brain. Acta Neuropathol |
|
| Host | RAB | |
| Species specificity | HU MS RT | |
| Figure 1 | 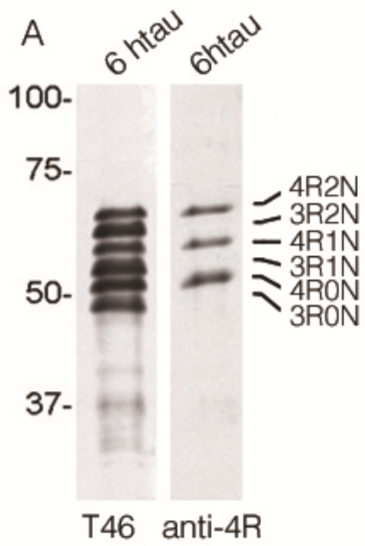 |
|
| Immunoblot analysis for 6 kinds of tau isoforms (3-repeat and 4-repeat) with anti total tau (T46) and Anti 4R (TIP-4RT-P01). | ||
| Figure 2 | 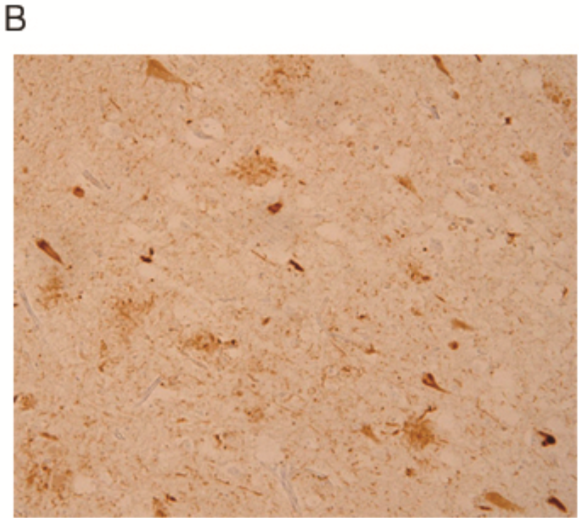 |
|
| Immunostaining for paraffin embedded section of cerebral cortex prepared from AD brain (retrieved with proteinase K and formic acid). | ||
| Product name | Anti Guanine Nucleotide Exchange C9orf72 (Poly-GA) pAb (Rabbit, Antiserum) | |
| Cat No | CAC-TIP-C9-P01 | |
| Description | In 20111 hexanucleotide expansions in the C9orf72 gene were identified in patients with frontotemporal lobar degeneration (FTLD) and Amyotrophic Lateral Sclerosis (ALS). GGGGCC expansions are characterized pathologically by the presence of TDP-43 negative and p62 positive inclusions in granule cells of cerebellum and in cells of the dentate gyrus and CA4 area of the hippocampus. It was reported that these inclusions include dipeptide repeat proteins, poly-GA, poly-GR and poly GP, arising from a putative non-ATG initiated sense translation of the GGGGCC expansion. Our C9orf72 antibodies are powerful tools for IHC analysis of neurodegenerative diseases. References: 1) David MA Mann, et al. Dipeptide repeat proteins are present in the p62 positive inclusions in patients with frontotemporal lobar degeneration and motor neuron disease associated with expansions in C9ORF72. Acta Neuropathologica Communications (2013) 1:68. PMID 24252525 2) Tan RH, et al. Cerebellar neuronal loss in als cases with ATXN2 intermediate repeat expansions. Ann Neurol. 2015 Nov 24. doi: 10.1002/ana.24565. PMID:26599997 3) Davidson Y, et al. Neurodegeneration in Frontotemporal Lobar Degeneration and Motor Neuron Disease associated with expansions in C9orf72 is linked to TDP-43 pathology and not associated with aggregated forms of dipeptide repeat proteins. Neuropathol Appl Neurobiol. 2015 Nov 5. doi: 10.1111/nan.12292. PMID: 26538301 4) Baborie A, et al. Accumulation of dipeptide repeat proteins predates that of TDP-43 in frontotemporal lobar degeneration associated with hexanucleotide repeat expansions in C9ORF72 gene. Neuropathol Appl Neurobiol. 2015 Aug;41(5):601-12. doi: 10.1111/nan.12178: 25185840 5) Davidson YS, et al. Brain distribution of dipeptide repeat proteins in frontotemporal lobar degeneration and motor neurone disease associated with expansions in C9ORF72. Acta Neuropathol Commun. 2014 Jun 20;2:70. doi: 10.1186/2051-5960-2-70. PMID: 24950788 6) Konno T, et al. C9ORF72 repeat-associated non-ATG-translated polypeptides are distributed independently of TDP-43 in a Japanese patient with c9ALS. Neuropathol Appl Neurobiol. 2014 Oct;40(6):783-8. doi: 10.1111/nan.12157. No abstract available. PMID: 24861677 |
|
| Host | RAB | |
| Species specificity | HU | |
| Figure 1 | 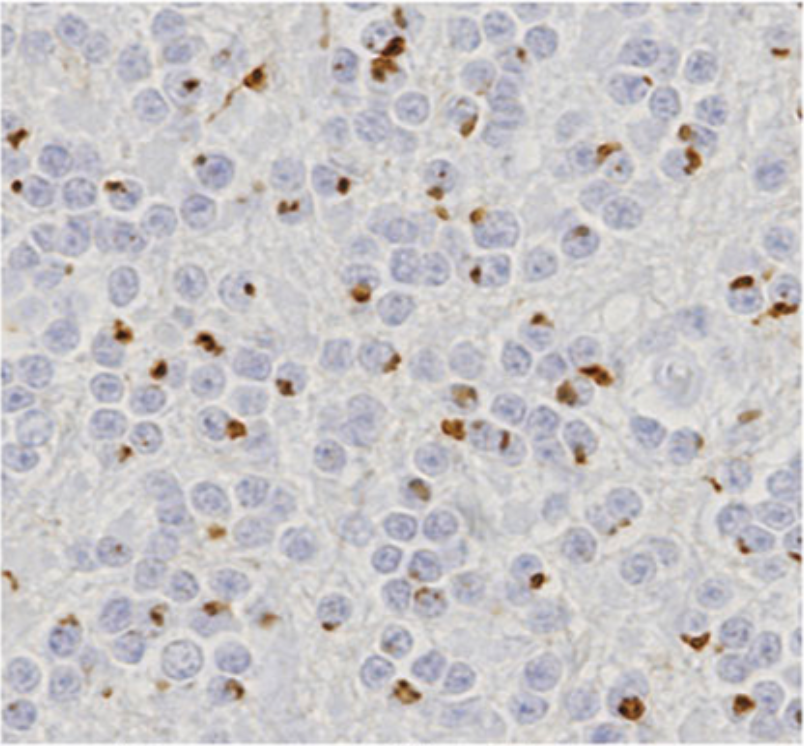 |
|
| Immunohistochemistry of C9orf72 detection with Poly GA antibody in cerebellum granular cell layer. | ||
| Product name | Anti Guanine Nucleotide Exchange C9orf72 (Poly-GR) pAb (Rabbit, Antiserum) | |
| Cat No | CAC-TIP-C9-P02 | |
| Description | In 20111 hexanucleotide expansions in the C9orf72 gene were identified in patients with frontotemporal lobar degeneration (FTLD) and Amyotrophic Lateral Sclerosis (ALS). GGGGCC expansions are characterized pathologically by the presence of TDP-43 negative and p62 positive inclusions in granule cells of cerebellum and in cells of the dentate gyrus and CA4 area of the hippocampus. It was reported that these inclusions include dipeptide repeat proteins, poly-GA, poly-GR and poly GP, arising from a putative non-ATG initiated sense translation of the GGGGCC expansion. Our C9orf72 antibodies are powerful tools for IHC analysis of neurodegenerative diseases. References: 1) David MA Mann, et al. Dipeptide repeat proteins are present in the p62 positive inclusions in patients with frontotemporal lobar degeneration and motor neuron disease associated with expansions in C9ORF72. Acta Neuropathologica Communications (2013) 1:68. PMID 24252525 2) Tan RH, et al. Cerebellar neuronal loss in als cases with ATXN2 intermediate repeat expansions. Ann Neurol. 2015 Nov 24. doi: 10.1002/ana.24565. PMID:26599997 3) Davidson Y, et al. Neurodegeneration in Frontotemporal Lobar Degeneration and Motor Neuron Disease associated with expansions in C9orf72is linked to TDP-43 pathology and not associated with aggregated forms of dipeptide repeat proteins. Neuropathol Appl Neurobiol. 2015 Nov 5. doi: 10.1111/nan.12292. PMID: 26538301 4) Baborie A, et al. Accumulation of dipeptide repeat proteins predates that of TDP-43 in frontotemporal lobar degeneration associated with hexanucleotide repeat expansions in C9ORF72 gene. Neuropathol Appl Neurobiol. 2015 Aug;41(5):601-12. doi: 10.1111/nan.12178. Epub 2015 Apr 30. PMID: 25185840 5) Davidson YS, et al. Brain distribution of dipeptide repeat proteins in frontotemporal lobar degeneration and motor neurone disease associated with expansions in C9ORF72. Acta Neuropathol Commun. 2014 Jun 20;2:70. doi: 10.1186/2051-5960-2-70. PMID: 24950788 6) Konno T, et al. C9ORF72 repeat-associated non-ATG-translated polypeptides are distributed independently of TDP-43 in a Japanese patient with c9ALS. Neuropathol Appl Neurobiol. 2014 Oct;40(6):783-8. doi: 10.1111/nan.12157. No abstract available. PMID: 24861677 |
|
| Host | RAB | |
| Species specificity | HU | |
| Product name | Anti Guanine Nucleotide Exchange C9orf72 (Poly-GP) pAb (Rabbit, Antiserum) | |
| Cat No | CAC-TIP-C9-P03 | |
| Description | In 20111 hexanucleotide expansions in the C9orf72 gene were identified in patients with frontotemporal lobar degeneration (FTLD) and Amyotrophic Lateral Sclerosis (ALS). GGGGCC expansions are characterized pathologically by the presence of TDP-43 negative and p62 positive inclusions in granule cells of cerebellum and in cells of the dentate gyrus and CA4 area of the hippocampus. It was reported that these inclusions include dipeptide repeat proteins, poly-GA, poly-GR and poly GP, arising from a putative non-ATG initiated sense translation of the GGGGCC expansion. Our C9orf72 antibodies are powerful tools for IHC analysis of neurodegenerative diseases. References: 1) David MA Mann, et al. Dipeptide repeat proteins are present in the p62 positive inclusions in patients with frontotemporal lobar degeneration and motor neuron disease associated with expansions in C9ORF72. Acta Neuropathologica Communications (2013) 1:68. PMID 24252525 2) Tan RH, et al. Cerebellar neuronal loss in als cases with ATXN2 intermediate repeat expansions. Ann Neurol. 2015 Nov 24. doi: 10.1002/ana.24565. PMID:26599997 3) Davidson Y, et al. Neurodegeneration in Frontotemporal Lobar Degeneration and Motor Neuron Disease associated with expansions in C9orf72is linked to TDP-43 pathology and not associated with aggregated forms of dipeptide repeat proteins. Neuropathol Appl Neurobiol. 2015 Nov 5. doi: 10.1111/nan.12292. PMID: 26538301 4) Baborie A, et al. Accumulation of dipeptide repeat proteins predates that of TDP-43 in frontotemporal lobar degeneration associated with hexanucleotide repeat expansions in C9ORF72 gene. Neuropathol Appl Neurobiol. 2015 Aug;41(5):601-12. doi: 10.1111/nan.12178. Epub 2015 Apr 30. PMID: 25185840 5) Davidson YS, et al. Brain distribution of dipeptide repeat proteins in frontotemporal lobar degeneration and motor neurone disease associated with expansions in C9ORF72. Acta Neuropathol Commun. 2014 Jun 20;2:70. doi: 10.1186/2051-5960-2-70. PMID: 24950788 6) Konno T, et al. C9ORF72 repeat-associated non-ATG-translated polypeptides are distributed independently of TDP-43 in a Japanese patient with c9ALS. Neuropathol Appl Neurobiol. 2014 Oct;40(6):783-8. doi: 10.1111/nan.12157. No abstract available. PMID: 24861677 |
|
| Host | RAB | |
| Species specificity | HU | |
| Product name | Anti TAR DNA-Binding Protein 43 (TDP-43), phospho Ser410 pAb (Rabbit, Antiserum) | |
| Cat No | CAC-TIP-PTD-P04 | |
| Description | TDP-43, a heterogeneous nuclear ribonucleoprotein, was identified as a component of ubiquitin-positive and tau-negative inclusions observed in cases of frontotemporal lobar degeneration (FTLD-U) and amyotrophic lateral sclerosis (ALS). Immunohistochemical analyses using antibodies generated against phospho- and non-phospho-peptides of human TDP-43 revealed that abnormally phosphorylated full-length TDP-43 (45 kDa), C-terminal fragments (~25 kDa) and smearing substances are deposited as intracellular inclusions in affected regions of FTLD-U and ALS cases. Our antibodies are powerful tools for biochemical and immunohistochemical analyses of neurodegenerative diseases and for evaluation of cellular or animal models of TDP-43 proteinopathy. References: Hasegawa M, Arai T, Nonaka T, et al. Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Ann Neurol. 2008;64(1):60–70. doi:10.1002/ana.21425 |
|
| Host | RAB | |
| Species specificity | HU | |
| Figure 1 | 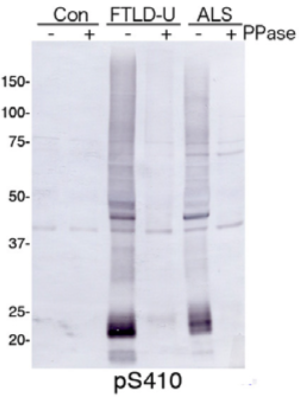 |
|
| Immunoblot analysis with pAb pS410. Predicted molecular weight: Phosphorylated full-length TDP-43 at 45 kDa, non-phosphorylated TDP-43 at 43 kDa, - 25 kDa fragments and smearing substances in frontotemporal lobar degeneration (FTLD-U) and ALS. |
||
| Figure 2 | 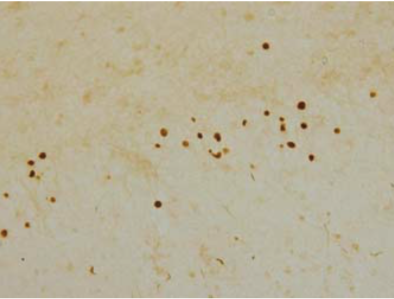 |
|
| Immunohistochemistry of TDP-43 lesions. pAb pS410 recognizes neuronal cytoplasmic inclusions and dystrophic neurites in dentate gyri of frontotemporal lobar degeneration (FTLD-U). |
||
| Product name | Anti TAR DNA-Binding Protein 43 (TDP-43), phospho Ser409 pAb (Rabbit, Antiserum) | |
| Cat No | CAC-TIP-PTD-P03 | |
| Description | TDP-43, a heterogeneous nuclear ribonucleoprotein, was identified as a component of ubiquitin-positive and tau-negative inclusions observed in cases of frontotemporal lobar degeneration (FTLD-U) and amyotrophic lateral sclerosis (ALS). Immunohistochemical analyses using antibodies generated against phospho- and non-phospho-peptides of human TDP-43 revealed that abnormally phosphorylated full-length TDP-43 (45 kDa), C-terminal fragments (~25 kDa) and smearing substances are deposited as intracellular inclusions in affected regions of FTLD-U and ALS cases. Our antibodies are powerful tools for biochemical and immunohistochemical analyses of neurodegenerative diseases and for evaluation of cellular or animal models of TDP-43 proteinopathy. References: Hasegawa M, Arai T, Nonaka T, et al. Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Ann Neurol. 2008;64(1):60–70. doi:10.1002/ana.21425 |
|
| Host | RAB | |
| Species specificity | HU | |
| Figure 1 | 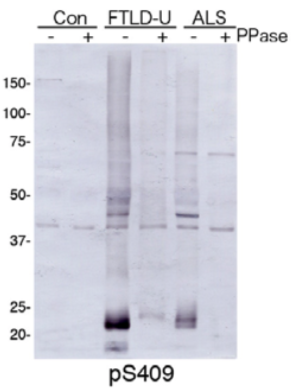 |
|
| Immunoblot analysis with pAb pS409. Predicted molecular weight: Phosphorylated full-length TDP-43 at 45 kDa, non-phosphorylated TDP-43 at 43 kDa, - 25 kDa fragments and smearing substances in frontotemporal lobar degeneration (FTLD-U) and ALS. |
||
| Figure 2 | 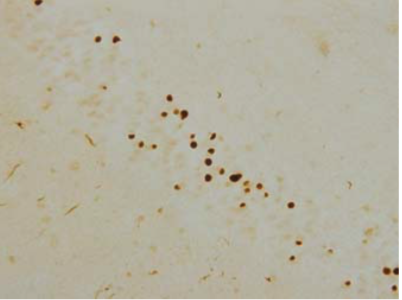 |
|
| Immunohistochemistry of TDP-43 lesions. pAb pS409 recognizes neuronal cytoplasmic inclusions and dystrophic neurites in dentate gyri of frontotemporal lobar degeneration (FTLD-U). |
||
| Product name | Anti TAR DNA-binding protein 43 (TDP-43) Amino Acids 3-12 pAb (Rabbit, Antiserum) | |
| Cat No | CAC-TIP-TD-P07 | |
| Description | TDP-43, a heterogeneous nuclear ribonucleoprotein, was identified as a component of ubiquitin-positive and tau-negative inclusions observed in cases of frontotemporal lobar degeneration (FTLD-U) and amyotrophic lateral sclerosis (ALS). Immunohistochemical analyses using antibodies generated against phospho- and non-phospho-peptides of human TDP-43 revealed that abnormally phosphorylated full-length TDP-43 (45 kDa), C-terminal fragments (~25 kDa) and smearing substances are deposited as intracellular inclusions in affected regions of FTLD-U and ALS cases. Our antibodies are powerful tools for biochemical and immunohistochemical analyses of neurodegenerative diseases and for evaluation of cellular or animal models of TDP-43 proteinopathy. References: Inukai, Yuki, Nonaka, Takashi, Arai, Tetsuaki, Yoshida, Mari, Hashizume, Yoshio, Beach, Thomas G., Buratti, Emanuele, Baralle, Francisco E., Akiyama, Haruhiko, Hisanaga, Shin-ichi and Hasegawa, Masato( 2008), Abnormal phosphorylation of Ser409/410 of TDP-43 in FTLD-U and ALS, FEBS Letters, 582, doi: 10.1016/j.febslet.2008.07.027 |
|
| Host | RAB | |
| Species specificity | HU RT | |
| Figure 1 | 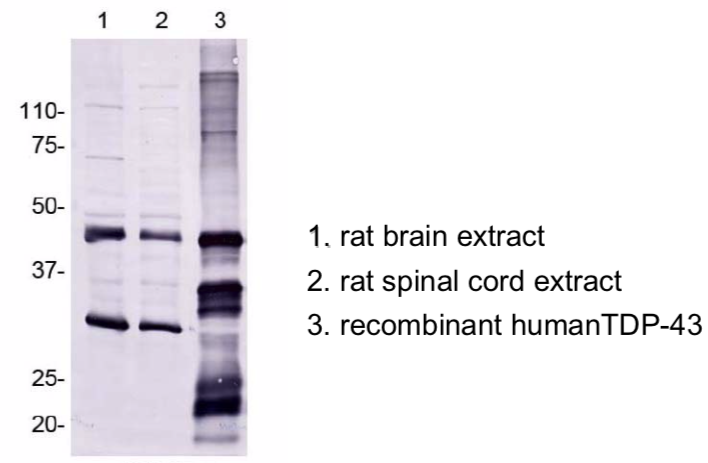 |
|
| Immunoblot analyses with pAb TDP43-N(3-12). Predicted molecular weight: Full-length TDP-43 at 43 kDa. | ||
| Figure 2 | 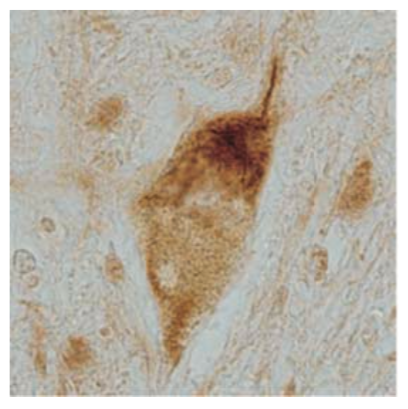 |
|
| Immunohistochemistry of skein-like inclusion in spinal cord of ALS. | ||
| Product name | Anti TAR DNA-binding protein 43 (TDP-43) Amino Acids 405-414 pAb (Rabbit, Antiserum) | |
| Cat No | CAC-TIP-TD-P09 | |
| Description | TDP-43, a heterogeneous nuclear ribonucleoprotein, was identified as a component of ubiquitin-positive and tau-negative inclusions observed in cases of frontotemporal lobar degeneration (FTLD-U) and amyotrophic lateral sclerosis (ALS). Immunohistochemical analyses using antibodies generated against phospho- and non-phospho-peptides of human TDP-43 revealed that abnormally phosphorylated full-length TDP-43 (45 kDa), C-terminal fragments (~25 kDa) and smearing substances are deposited as intracellular inclusions in affected regions of FTLD-U and ALS cases. Our antibodies are powerful tools for biochemical and immunohistochemical analyses of neurodegenerative diseases and for evaluation of cellular or animal models of TDP-43 proteinopathy. References: Inukai, Yuki, Nonaka, Takashi, Arai, Tetsuaki, Yoshida, Mari, Hashizume, Yoshio, Beach, Thomas G., Buratti, Emanuele, Baralle, Francisco E., Akiyama, Haruhiko, Hisanaga, Shin-ichi and Hasegawa, Masato( 2008), Abnormal phosphorylation of Ser409/410 of TDP-43 in FTLD-U and ALS, FEBS Letters, 582, doi: 10.1016/j.febslet.2008.07.027 |
|
| Host | RAB | |
| Species specificity | HU RT | |
| Figure 1 | 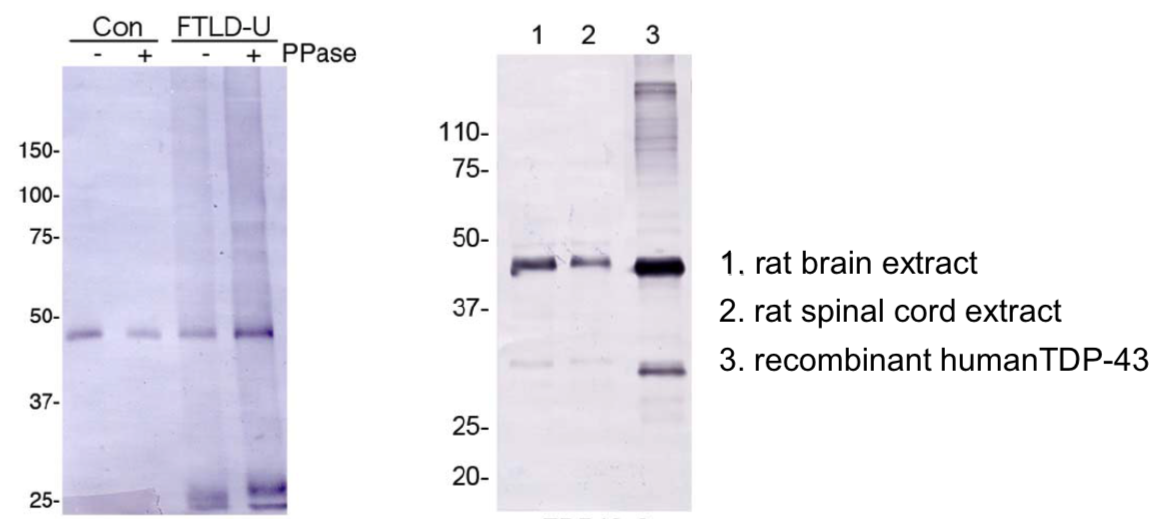 |
|
| Predicted molecular weight: Full-length TDP-43 at 43 kDa. | ||
| Figure 2 | 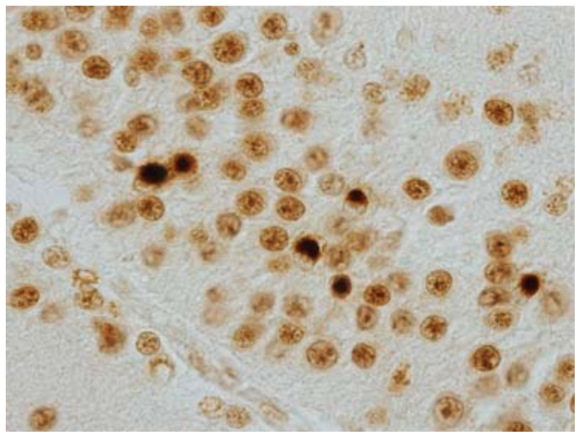 |
|
| Immunohistochemistry of Nuclei and NCIs in dentate gyrus of FTLD-U. | ||
| Product name | Anti Amyloid-Beta Precursor Protein (APP) mAb (Clone 278) | |
| Cat No | CAC-YCU-MK-AP01 | |
| Description | Amyloid precursor protein (APP) is an integral membrane protein expressed in many tissues and concentrated in the synapses of neurons. Its primary function is not known, though it has been implicated as a regulator of synapse formation, neural plasticity, antimicrobial activity, and iron export. APP is best known as the precursor molecule whose proteolysis generates beta amyloid (Aβ), a polypeptide containing 37 to 49 amino acid residues, whose amyloid fibrillar form is the primary component of amyloid plaques found in the brains of Alzheimer's disease patients. [Amyloid precursor protein, https://en.wikipedia.org/w/index.php?title=Amyloid_precursor_protein&oldid=917190737 (last visited Oct. 2, 2019).] References: 1) Higashi, S. and Miyazaki, K. Novel processing of β-amyloid precursor protein catalyzed by membrane type1 matrix metalloproteinase releases a fragment lacking the inhibitor domain against gelatinase A. Biochemistry, 42: 6514-6526, 2003. 2) Higashi, S., Miyazaki, K.: Identification of a region of beta-amyloid precursor protein essential for its gelatinase A inhibitory activity. J Biol Chem. 278(16): 14020-14028, 2003. 3) Miyata, S., Koshikawa, N., Higashi, S., Miyagi, Y., Nagashima, Y., Yanoma, S., Kato, Y., Yasumitsu H., Miyazaki, K. Expression of trypsin in human cancer cell lines and cancer tissues and its tight binding to soluble form of Alzheimer amyloid precursor protein in culture. J Biochem. 125(6): 1067-1076, 1999. 4) Koshikawa,N., Nakamura,T., Tsuchiya,N., Isaji,M., Yasumitsu,H., Umeda,M., Miyazaki,K. Purification and identification of a novel and four known serine proteinase inhibitors secreted by human glioblastoma cells. J Biochem. 119(2): 334-339, 1996. |
|
| Host | MS | |
| Species specificity | HU | |
| Figure 1 | 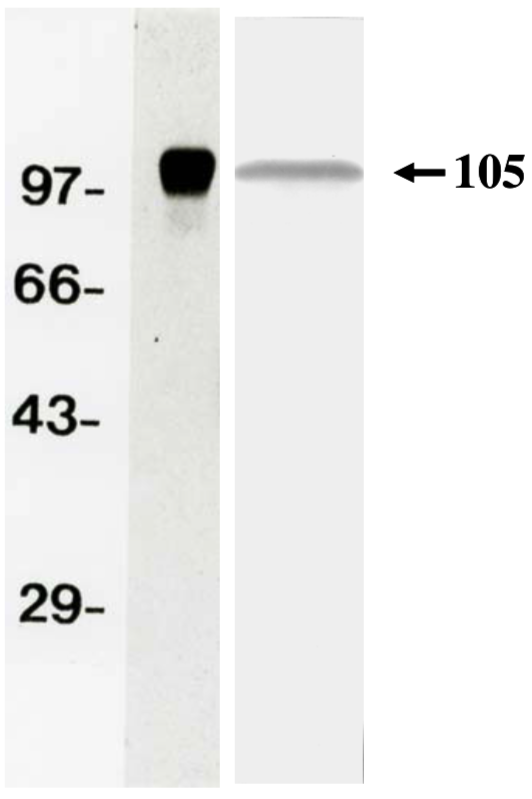 |
|
| Western Blot Analysis for purified APP (soluble) and supernatant of Human Fibrosarcoma Cell Line HT1080 (left). | ||
| Product name | Anti Alpha Synuclein (Amino Acids 1-10) pAb (Rabbit, Antiserum) | |
| Cat No | CAC-TIP-SN-P01 | |
| Description | Alpha-Synuclein, a 140-amino acid protein abundantly expressed in presynaptic terminals, is a component of intraneuronal or glial inclusions observed in cases of Parkinson's disease (PD), Dementia with Lewy bodies (DLB) and Multiple system atrophy (MSA). Although alpha-synuclein is a natively unfolded protein, fibrillization or conformational change(s) of alpha-synuclein is central to the pathogenesis of alpha-synucleinopathies. The amino-terminal region of alpha-synuclein consists of seven imperfect repeats, each 11 amino acids in length, with the consensus sequence KTKEGV. The repeats partially overlap with a hydrophobic region (amino acids 61-95). The carboxy-terminal region (amino acids 96-140) is negatively charged. These antibodies are powerful tools for biochemical and IHC analyses of neurodegenerative diseases and for evaluation of conformational changes of alpha-synuclein. References: 1) Masami Masuda et al., Inhibition of a-synuclein fibril assembly by small molecules: Analysis using epitope-specific antibodies. FEBS Letters (2009) 583, 787-791. PMID 19183551 2) Motokuni Yonetani et al., Conversion of wild-type alpha-synuclein into mutant-type fibrils and its propagation in the presence of A30P mutant. Journal of Biological Chemistry (2009) 284, 7940-7950. PMID 19164293 |
|
| Host | RAB | |
| Species specificity | HU MS | |
| Product name | Anti Alpha Synuclein (Amino Acids 11-20) pAb (Rabbit, Antiserum) | |
| Cat No | CAC-TIP-SN-P02 | |
| Description | Alpha-Synuclein, a 140-amino acid protein abundantly expressed in presynaptic terminals, is a component of intraneuronal or glial inclusions observed in cases of Parkinson's disease (PD), Dementia with Lewy bodies (DLB) and Multiple system atrophy (MSA). Although alpha-synuclein is a natively unfolded protein, fibrillization or conformational change(s) of alpha-synuclein is central to the pathogenesis of alpha-synucleinopathies. The amino-terminal region of alpha-synuclein consists of seven imperfect repeats, each 11 amino acids in length, with the consensus sequence KTKEGV. The repeats partially overlap with a hydrophobic region (amino acids 61-95). The carboxy-terminal region (amino acids 96-140) is negatively charged. These antibodies are powerful tools for biochemical and IHC analyses of neurodegenerative diseases and for evaluation of conformational changes of alpha-synuclein. References: 1) Masami Masuda et al., Inhibition of a-synuclein fibril assembly by small molecules: Analysis using epitope-specific antibodies. FEBS Letters (2009) 583, 787-791. PMID 19183551 2) Motokuni Yonetani et al., Conversion of wild-type alpha-synuclein into mutant-type fibrils and its propagation in the presence of A30P mutant. Journal of Biological Chemistry (2009) 284, 7940-7950. PMID 19164293 |
|
| Host | RAB | |
| Species specificity | HU MS | |
| Product name | Anti Alpha Synuclein (Amino Acids 21-30) pAb (Rabbit, Antiserum) | |
| Cat No | CAC-TIP-SN-P03 | |
| Description | Alpha-Synuclein, a 140-amino acid protein abundantly expressed in presynaptic terminals, is a component of intraneuronal or glial inclusions observed in cases of Parkinson's disease (PD), Dementia with Lewy bodies (DLB) and Multiple system atrophy (MSA). Although alpha-synuclein is a natively unfolded protein, fibrillization or conformational change(s) of alpha-synuclein is central to the pathogenesis of alpha-synucleinopathies. The amino-terminal region of alpha-synuclein consists of seven imperfect repeats, each 11 amino acids in length, with the consensus sequence KTKEGV. The repeats partially overlap with a hydrophobic region (amino acids 61-95). The carboxy-terminal region (amino acids 96-140) is negatively charged. These antibodies are powerful tools for biochemical and IHC analyses of neurodegenerative diseases and for evaluation of conformational changes of alpha-synuclein. References: 1) Masami Masuda et al., Inhibition of a-synuclein fibril assembly by small molecules: Analysis using epitope-specific antibodies. FEBS Letters (2009) 583, 787-791. PMID 19183551 2) Motokuni Yonetani et al., Conversion of wild-type alpha-synuclein into mutant-type fibrils and its propagation in the presence of A30P mutant. Journal of Biological Chemistry (2009) 284, 7940-7950. PMID 19164293 |
|
| Host | RAB | |
| Species specificity | HU MS | |
| Product name | Anti Alpha Synuclein (Amino Acids 31-40) pAb (Rabbit, Antiserum) | |
| Cat No | CAC-TIP-SN-P04 | |
| Description | Alpha-Synuclein, a 140-amino acid protein abundantly expressed in presynaptic terminals, is a component of intraneuronal or glial inclusions observed in cases of Parkinson's disease (PD), Dementia with Lewy bodies (DLB) and Multiple system atrophy (MSA). Although alpha-synuclein is a natively unfolded protein, fibrillization or conformational change(s) of alpha-synuclein is central to the pathogenesis of alpha-synucleinopathies. The amino-terminal region of alpha-synuclein consists of seven imperfect repeats, each 11 amino acids in length, with the consensus sequence KTKEGV. The repeats partially overlap with a hydrophobic region (amino acids 61-95). The carboxy-terminal region (amino acids 96-140) is negatively charged. These antibodies are powerful tools for biochemical and IHC analyses of neurodegenerative diseases and for evaluation of conformational changes of alpha-synuclein. References: 1) Masami Masuda et al., Inhibition of a-synuclein fibril assembly by small molecules: Analysis using epitope-specific antibodies. FEBS Letters (2009) 583, 787-791. PMID 19183551 2) Motokuni Yonetani et al., Conversion of wild-type alpha-synuclein into mutant-type fibrils and its propagation in the presence of A30P mutant. Journal of Biological Chemistry (2009) 284, 7940-7950. PMID 19164293 |
|
| Host | RAB | |
| Species specificity | HU MS | |
| Product name | Anti Alpha Synuclein (Amino Acids 41-50) pAb (Rabbit, Antiserum) | |
| Cat No | CAC-TIP-SN-P05 | |
| Description | Alpha-Synuclein, a 140-amino acid protein abundantly expressed in presynaptic terminals, is a component of intraneuronal or glial inclusions observed in cases of Parkinson's disease (PD), Dementia with Lewy bodies (DLB) and Multiple system atrophy (MSA). Although alpha-synuclein is a natively unfolded protein, fibrillization or conformational change(s) of alpha-synuclein is central to the pathogenesis of alpha-synucleinopathies. The amino-terminal region of alpha-synuclein consists of seven imperfect repeats, each 11 amino acids in length, with the consensus sequence KTKEGV. The repeats partially overlap with a hydrophobic region (amino acids 61-95). The carboxy-terminal region (amino acids 96-140) is negatively charged. These antibodies are powerful tools for biochemical and IHC analyses of neurodegenerative diseases and for evaluation of conformational changes of alpha-synuclein. References: 1) Masami Masuda et al., Inhibition of a-synuclein fibril assembly by small molecules: Analysis using epitope-specific antibodies. FEBS Letters (2009) 583, 787-791. PMID 19183551 2) Motokuni Yonetani et al., Conversion of wild-type alpha-synuclein into mutant-type fibrils and its propagation in the presence of A30P mutant. Journal of Biological Chemistry (2009) 284, 7940-7950. PMID 19164293 |
|
| Host | RAB | |
| Species specificity | HU MS | |
| Product name | Anti Alpha Synuclein (Amino Acids 51-60) pAb (Rabbit, Antiserum) | |
| Cat No | CAC-TIP-SN-P06 | |
| Description | Alpha-Synuclein, a 140-amino acid protein abundantly expressed in presynaptic terminals, is a component of intraneuronal or glial inclusions observed in cases of Parkinson's disease (PD), Dementia with Lewy bodies (DLB) and Multiple system atrophy (MSA). Although alpha-synuclein is a natively unfolded protein, fibrillization or conformational change(s) of alpha-synuclein is central to the pathogenesis of alpha-synucleinopathies. The amino-terminal region of alpha-synuclein consists of seven imperfect repeats, each 11 amino acids in length, with the consensus sequence KTKEGV. The repeats partially overlap with a hydrophobic region (amino acids 61-95). The carboxy-terminal region (amino acids 96-140) is negatively charged. These antibodies are powerful tools for biochemical and IHC analyses of neurodegenerative diseases and for evaluation of conformational changes of alpha-synuclein. References: 1) Masami Masuda et al., Inhibition of a-synuclein fibril assembly by small molecules: Analysis using epitope-specific antibodies. FEBS Letters (2009) 583, 787-791. PMID 19183551 2) Motokuni Yonetani et al., Conversion of wild-type alpha-synuclein into mutant-type fibrils and its propagation in the presence of A30P mutant. Journal of Biological Chemistry (2009) 284, 7940-7950. PMID 19164293 |
|
| Host | RAB | |
| Species specificity | HU MS | |
| Product name | Anti Alpha Synuclein (Amino Acids 61-70) pAb (Rabbit, Antiserum) | |
| Cat No | CAC-TIP-SN-P07 | |
| Description | Alpha-Synuclein, a 140-amino acid protein abundantly expressed in presynaptic terminals, is a component of intraneuronal or glial inclusions observed in cases of Parkinson's disease (PD), Dementia with Lewy bodies (DLB) and Multiple system atrophy (MSA). Although alpha-synuclein is a natively unfolded protein, fibrillization or conformational change(s) of alpha-synuclein is central to the pathogenesis of alpha-synucleinopathies. The amino-terminal region of alpha-synuclein consists of seven imperfect repeats, each 11 amino acids in length, with the consensus sequence KTKEGV. The repeats partially overlap with a hydrophobic region (amino acids 61-95). The carboxy-terminal region (amino acids 96-140) is negatively charged. These antibodies are powerful tools for biochemical and IHC analyses of neurodegenerative diseases and for evaluation of conformational changes of alpha-synuclein. References: 1) Masami Masuda et al., Inhibition of a-synuclein fibril assembly by small molecules: Analysis using epitope-specific antibodies. FEBS Letters (2009) 583, 787-791. PMID 19183551 2) Motokuni Yonetani et al., Conversion of wild-type alpha-synuclein into mutant-type fibrils and its propagation in the presence of A30P mutant. Journal of Biological Chemistry (2009) 284, 7940-7950. PMID 19164293 |
|
| Host | RAB | |
| Species specificity | HU MS | |
| Product name | Anti Alpha Synuclein (Amino Acids 75-91) pAb (Rabbit, Antiserum) | |
| Cat No | CAC-TIP-SN-P08 | |
| Description | Alpha-Synuclein, a 140-amino acid protein abundantly expressed in presynaptic terminals, is a component of intraneuronal or glial inclusions observed in cases of Parkinson's disease (PD), Dementia with Lewy bodies (DLB) and Multiple system atrophy (MSA). Although alpha-synuclein is a natively unfolded protein, fibrillization or conformational change(s) of alpha-synuclein is central to the pathogenesis of alpha-synucleinopathies. The amino-terminal region of alpha-synuclein consists of seven imperfect repeats, each 11 amino acids in length, with the consensus sequence KTKEGV. The repeats partially overlap with a hydrophobic region (amino acids 61-95). The carboxy-terminal region (amino acids 96-140) is negatively charged. These antibodies are powerful tools for biochemical and IHC analyses of neurodegenerative diseases and for evaluation of conformational changes of alpha-synuclein. References: 1) Masami Masuda et al., Inhibition of a-synuclein fibril assembly by small molecules: Analysis using epitope-specific antibodies. FEBS Letters (2009) 583, 787-791. PMID 19183551 2) Motokuni Yonetani et al., Conversion of wild-type alpha-synuclein into mutant-type fibrils and its propagation in the presence of A30P mutant. Journal of Biological Chemistry (2009) 284, 7940-7950. PMID 19164293 |
|
| Host | RAB | |
| Species specificity | HU MS | |
| Product name | Anti Alpha Synuclein (Amino Acids 131-140) pAb (Rabbit, Antiserum) | |
| Cat No | CAC-TIP-SN-P09 | |
| Description | Alpha-Synuclein, a 140-amino acid protein abundantly expressed in presynaptic terminals, is a component of intraneuronal or glial inclusions observed in cases of Parkinson's disease (PD), Dementia with Lewy bodies (DLB) and Multiple system atrophy (MSA). Although alpha-synuclein is a natively unfolded protein, fibrillization or conformational change(s) of alpha-synuclein is central to the pathogenesis of alpha-synucleinopathies. The amino-terminal region of alpha-synuclein consists of seven imperfect repeats, each 11 amino acids in length, with the consensus sequence KTKEGV. The repeats partially overlap with a hydrophobic region (amino acids 61-95). The carboxy-terminal region (amino acids 96-140) is negatively charged. These antibodies are powerful tools for biochemical and IHC analyses of neurodegenerative diseases and for evaluation of conformational changes of alpha-synuclein. References: 1) Masami Masuda et al., Inhibition of a-synuclein fibril assembly by small molecules: Analysis using epitope-specific antibodies. FEBS Letters (2009) 583, 787-791. PMID 19183551 2) Motokuni Yonetani et al., Conversion of wild-type alpha-synuclein into mutant-type fibrils and its propagation in the presence of A30P mutant. Journal of Biological Chemistry (2009) 284, 7940-7950. PMID 19164293 |
|
| Host | RAB | |
| Species specificity | HU MS | |
| Product name | Anti Alpha Synuclein (9 Antibody Set) pAb (Rabbit, Antiserum) | |
| Cat No | CAC-TIP-SN-SET | |
| Description | Alpha-synuclein, a 140-amino acid protein abundantly expressed in presynaptic terminals, is a component of intraneuronal or glial inclusions observed in cases of Parkinson's disease (PD), Dementia with Lewy bodies (DLB) and Multiple system atrophy (MSA). Although alpha-synuclein is a natively unfolded protein, fibrillization or conformational change(s) of alpha-synuclein is central to the pathogenesis of alpha-synucleinopathies. The amino-terminal region of alpha-synuclein consists of seven imperfect repeats, each 11 amino acids in length, with the consensus sequence KTKEGV. The repeats partially overlap with a hydrophobic region (amino acids 61-95). The carboxy-terminal region (amino acids 96-140) is negatively charged. These antibodies are powerful tools for biochemical and IHC analyses of neurodegenerative diseases and for evaluation of conformational changes of alpha-synuclein. References: 1) Masami Masuda et al., Inhibition of a-synuclein fibril assembly by small molecules: Analysis using epitope-specific antibodies. FEBS Letters (2009) 583, 787-791. PMID 19183551 2) Motokuni Yonetani et al., Conversion of wild-type alpha-synuclein into mutant-type fibrils and its propagation in the presence of A30P mutant. Journal of Biological Chemistry (2009) 284, 7940-7950. PMID 19164293 |
|
| Host | RAB | |
| Species specificity | HU MS | |
| Product name | Anti Parvalbumin Alpha pAb (Rabbit, Affinity Purified) | |
| Cat No | CAC-ACC-PA001 | |
| Description | Parvalbumin (PV) is a calcium binding protein expressed in specific muscle fibers and fast-firing neurons. PV consists of a single, unbranched chain of linked amino acids and belongs to a larger group of EF hand proteins. Studies have demonstrated that parvalbumin acts in the decay of calcium in the contraction/relaxation cycle of fast twitch muscles. This data has shown a positive correlation between the rate of relaxation and the concentration of parvalbumin. Parvalbumin is also expressed in a specific population of GABAergic interneurons which are thought to play a role in maintaining the balance between excitation and inhibition in the cortex as well as the hippocampus. In amyotrophic lateral sclerosis (ALS) patents, parvalbumin immunoreactivity is specifically absent from neuron populations lost early in ALS. References: Inaguma Y, Kurobe N, Shinohara H, Kato K. (1991) Sensitive immunoassay for rat parvalbumin: tissue distribution and developmental changes. Biochim Biophys Acta. 1075: 68-74. |
|
| Host | RAB | |
| Species specificity | HU RT | |
| Product name | Anti Protein S100-A1 (S100 Alpha) pAb (Rabbit, Affinity Purified) | |
| Cat No | CAC-ACC-PA002 | |
| Description | The S100 protein is a low-molecular-weight, acidic and calcium binding protein that in functional form exist in dimers. S100 has two subunits: S100-alpha (94 aa; human chromosome 1) and S100-beta (92 aa; human chromosome 21) that forms as either homodimers (alpha-alpha known as S-100a(0) or beta-beta known as S-100b) or as heterodimers (known as S-100a) of ~21 kDa. S100-alpha and -beta chains show ~58% sequence identity and are both highly conserved among species. S100-alpha was originally believed to be localized to the CNS, but studies have shown it to be found in numerous tissues including cardiac, skeletal and vascular smooth muscle cells. References: Kato K, Haimoto H, Ariyoshi Y, Horisawa M, Washida H, Kimura S. (1985) High levels of S-100a0 (alpha alpha) protein in tumor tissues and in sera of patients with renal cell carcinoma. Jpn J Cancer Res. 76:856-62. |
|
| Host | RAB | |
| Species specificity | HU MS RT BOV POR | |
| Product name | Anti Calbindin (D-28K) pAb (Rabbit, Affinity Purified) | |
| Cat No | CAC-ACC-PA003 | |
| Description | Vitamin D-dependent 28 kDa calcium-binding protein (Calbindin D 28k or CB) is a member of the calmodulin superfamily of calcium binding proteins discovered in 1966 by Wasserman and Taylor. The protein has a distinct distribution in the brain and sensory system and is abundant in specific neuronal cell types. Calbindin D-28k constitutes as much as 0.1-1.5 % of the total soluble protein in brain and it may be present at intracellular concentrations up to 1 mM. Several recent studies have shown that calbindin D can protect cells from degeneration and ischemic injury under extracellular stress. In particular, this is demonstrated in the brain of Alzheimer and quotes disease patients. Cells containing calbindin D have a considerably lower plaque and tangles burden than those lacking this 28 kDa protein. References: Zhu YY, Takashi M, Miyake K, Kato K. (1991) Sensitive enzyme immunoassay for human 28 kDa calbindin-D. Clin Chim Acta. 1991 201:183-92. |
|
| Host | RAB | |
| Species specificity | HU MS RT | |
| Product name | Anti Alpha-Crystallin B Chain, p19S pAb (Rabbit, Affinity Purified) | |
| Cat No | CAC-ACC-PA004 | |
| Description | Lens proteins consist almost entirely of crystallins (about 95%). Crystallins are also found in vertebrate skeletal muscle tissue. In the lens, their structural function is to assist in maintaining the proper refractive index of the lens. The mammalian lens contains 3 major classes of crystallins: alpha, beta, and gamma. Alpha-crystallin is the largest of the crystallins and is composed of 2 primary gene products, alpha-A and alpha-B. There are at least 5 different proteins comprising the beta-crystallins. The gamma-crystallins are monomeric, but there are at least 5 gamma crystallins identified in bovine and rat lens. Alpha-Crystallin comprises 40% of total lens protein composition. In addition to maintaining proper refractive index, it also functions in a chaperone like manner by preventing the formation of aggregates possibly leading to cataract formation. It is believed that the phosphorylated states of the alpha-crystallin occur in response to cellular stress and may serve a structural control function and play a role in protein maintenance. Alpha-B crystallin has been linked to Alexander and quotes disease where it accumulates in brain cells of those afflicted. References: 1) Ito H, Okamoto K, Nakayama H, Isobe T, Kato K. (1997) Phosphorylation of B-crystallin in response to various types of stress. J Biol Chem. 272, 29934-29941. 2) Kato K, Ito H, Kamei K, Inaguma Y, Iwamoto I, Saga S. (1998) Phosphorylation of B-crystallin in mitotic cells and identification of enzymatic activities responsible for phosphorylation. J Biol Chem. 273, 28346-28354. 3) Ito H, Iida K, Kamei K, Iwamoto I, Inaguma Y, Kato K. (1999) B-crystallin in rat lens is phosphorylated at an early postnatal age. FEBS Lett. 446, 269-272. |
|
| Host | RAB | |
| Species specificity | HU MS RT BOV | |
| Product name | Anti Alpha-Crystallin B Chain, p45S pAb (Rabbit, Affinity Purified) | |
| Cat No | CAC-ACC-PA005 | |
| Description | Lens proteins consist almost entirely of crystallins (about 95%). Crystallins are also found in vertebrate skeletal muscle tissue. In the lens, their structural function is to assist in maintaining the proper refractive index of the lens. The mammalian lens contains 3 major classes of crystallins: alpha, beta, and gamma. Alpha-crystallin is the largest of the crystallins and is composed of 2 primary gene products, alpha-A and alpha-B. There are at least 5 different proteins comprising the beta-crystallins. The gamma-crystallins are monomeric, but there are at least 5 gamma crystallins identified in bovine and rat lens. Alpha-Crystallin comprises 40% of total lens protein composition. In addition to maintaining proper refractive index, it also functions in a chaperone like manner by preventing the formation of aggregates possibly leading to cataract formation. It is believed that the phosphorylated states of the alpha-crystallin occur in response to cellular stress and may serve a structural control function and play a role in protein maintenance. Alpha-B crystallin has been linked to Alexander and quotes disease where it accumulates in brain cells of those afflicted. References: 1) Ito H, Okamoto K, Nakayama H, Isobe T, Kato K. (1997) Phosphorylation of B-crystallin in response to various types of stress. J Biol Chem. 272, 29934-29941. 2) Kato K, Ito H, Kamei K, Inaguma Y, Iwamoto I, Saga S. (1998) Phosphorylation of B-crystallin in mitotic cells and identification of enzymatic activities responsible for phosphorylation. J Biol Chem. 273, 28346-28354. 3) Ito H, Iida K, Kamei K, Iwamoto I, Inaguma Y, Kato K. (1999) B-crystallin in rat lens is phosphorylated at an early postnatal age. FEBS Lett. 446, 269-272. |
|
| Host | RAB | |
| Species specificity | HU MS RT BOV | |
| Figure 1 | 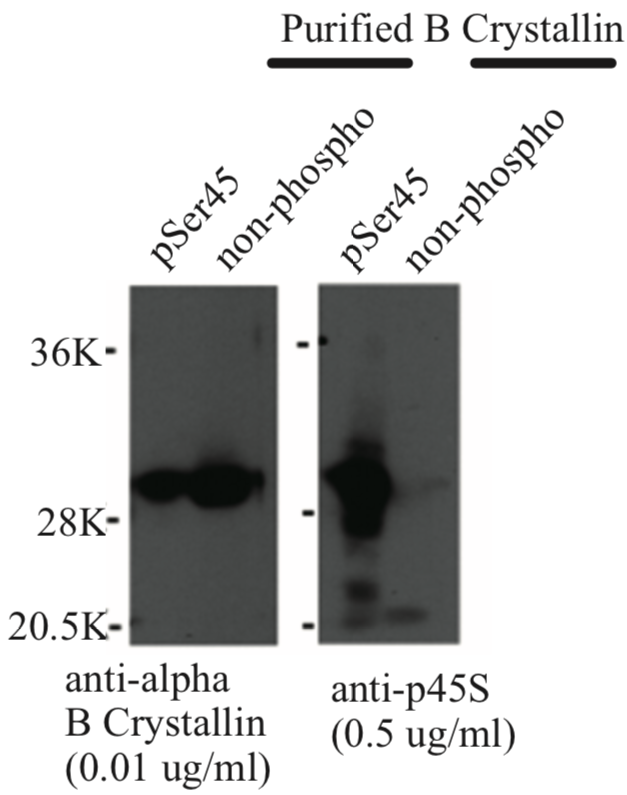 |
|
| Immunoblot analysis of anti-alpha-B crystallin p45S antibody. | ||
| Product name | Anti Alpha-Crystallin B Chain, p59S pAb (Rabbit, Affinity Purified) | |
| Cat No | CAC-ACC-PA006 | |
| Description | Lens proteins consist almost entirely of crystallins (about 95%). Crystallins are also found in vertebrate skeletal muscle tissue. In the lens, their structural function is to assist in maintaining the proper refractive index of the lens. The mammalian lens contains 3 major classes of crystallins: alpha, beta, and gamma. Alpha-crystallin is the largest of the crystallins and is composed of 2 primary gene products, alpha-A and alpha-B. There are at least 5 different proteins comprising the beta-crystallins. The gamma-crystallins are monomeric, but there are at least 5 gamma crystallins identified in bovine and rat lens. Alpha-Crystallin comprises 40% of total lens protein composition. In addition to maintaining proper refractive index, it also functions in a chaperone like manner by preventing the formation of aggregates possibly leading to cataract formation. It is believed that the phosphorylated states of the alpha-crystallin occur in response to cellular stress and may serve a structural control function and play a role in protein maintenance. Alpha-B crystallin has been linked to Alexander and quotes disease where it accumulates in brain cells of those afflicted. References: 1) Ito H, Okamoto K, Nakayama H, Isobe T, Kato K. (1997) Phosphorylation of B-crystallin in response to various types of stress. J Biol Chem. 272, 29934-29941. 2) Kato K, Ito H, Kamei K, Inaguma Y, Iwamoto I, Saga S. (1998) Phosphorylation of B-crystallin in mitotic cells and identification of enzymatic activities responsible for phosphorylation. J Biol Chem. 273, 28346-28354. 3) Ito H, Iida K, Kamei K, Iwamoto I, Inaguma Y, Kato K. (1999) B-crystallin in rat lens is phosphorylated at an early postnatal age. FEBS Lett. 446, 269-272. |
|
| Host | RAB | |
| Species specificity | HU RT BOV | |
| Figure 1 | 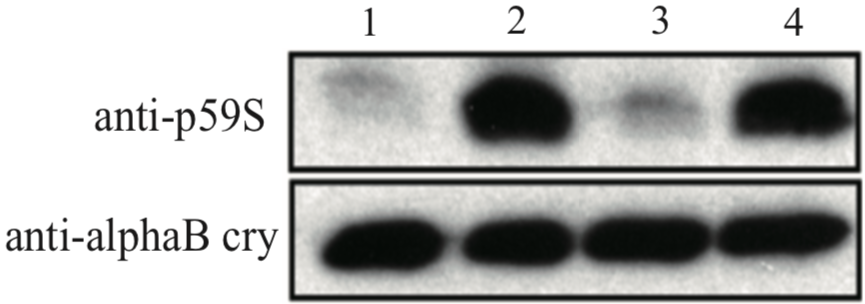 |
|
| Immunoblot analysis with anti alpha-B crystallin p59S antibody. U373MG cell lysates 1: control 2: 4mM H2O2 for 90min 3: 4mM H2O2 + 10uM SB203580 for 90min 4: 4mM H2O2 + 30uM SB203580 for 90min |
||
| Product name | Anti Dysbindin (Dystrobrevin-Binding Protein 1) pAb (Rabbit, Affinity Purified) | |
| Cat No | CAC-ACC-PA007 | |
| Description | Dysbindin is a component of the BLOC-1 complex, a complex that is required for normal biogenesis of lysosome-related organelles (LRO), such as platelet dense granules and melanosomes. In concert with the AP-3 complex, the BLOC-1 complex is required to target membrane protein cargos into vesicles assembled at cell bodies for delivery into neurites and nerve terminals. The BLOC-1 complex, in association with SNARE proteins, is also proposed to be involved in neurite extension. It plays a role in synaptic vesicle trafficking and in neurotransmitter release. 3 isoforms produced by alternative splicing and alternative initiation have been described. Isoform 1 is mainly cytoplasmic, but shuttles between the cytoplasm and nucleus. It is exported out of the nucleus via its nuclear export sequence (NES). Its nuclear localization is required for regulation of the expression of genes, such as SYN1. It is detected in neuron cell bodies, axons and dendrites and is mainly located to the postsynaptic density. Isoform 2 also shuttles between the cytoplasm and nucleus and is mainly expressed in the dendritic spine. It is predominantly a synaptic vesicle isoform but also highly expressed in the nucleus. Isoform 3 is exclusively cytoplasmic and is predominantly found in the postsynaptic density (PSD) with little association with synaptic vesicles. Mutations in DTNBP1 gene have been linked to Hermansky-Pudlak syndrome 7 (HPS7) that is characterized by oculocutaneous albinism, bleeding due to platelet storage pool deficiency, and lysosomal storage defects. Defects in DTNBP1 are associated with susceptibility to schizophrenia. References: 1) Ito H, Morishita R, Shinoda T, Iwamoto I, Sudo K, Okamoto K, Nagata KI. (2010) Dysbindin-1, WAVE2 and Abi-1 form a complex that regulates dendritic spine formation. Mol Psychiatry 15:976-986. 2) Ito H, Morishita R, Nagata K. (2016) Schizophrenia susceptibility gene product dysbindin-1 regulates the homeostasis of cyclin D1. Biochim Biophys Acta. 1862: 1383-1391. |
|
| Host | RAB | |
| Species specificity | HU MS RT | |
| Figure 1 | 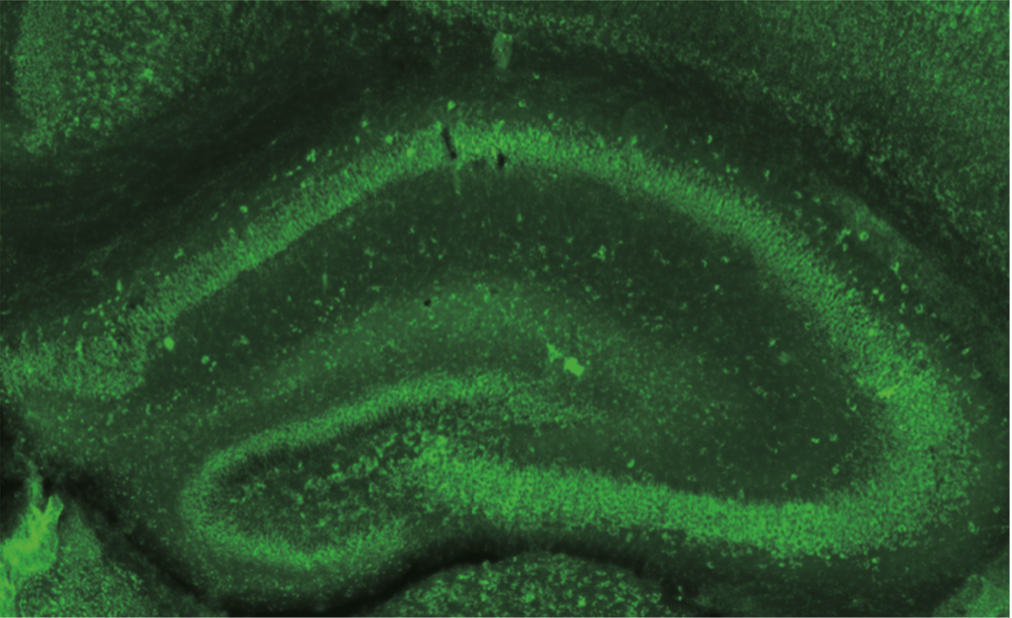 |
|
| Immunohistochemistry of formalin/PFA-fixed paraffin embedded sections stained with Anti Dysbindin-1 antibody. | ||
| Figure 2 | 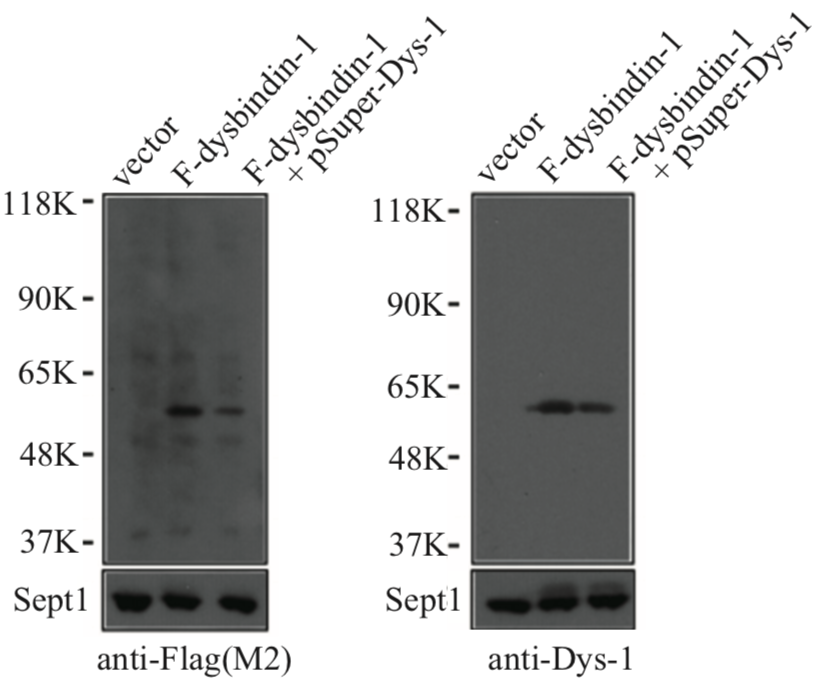 |
|
| Immunoblot analysis with anti Dysbindin-1 antibody. | ||
| Product name | Anti Inter-Alpha-Trypsin Inhibitor Heavy Chain H4 (ITIH4) pAb (Brown Norway Rat, Antiserum) | |
| Cat No | CAC-ICA-TG2-RTP1 | |
| Description | ITIH4 is secreted into the blood and is cleaved by plasma kallikrein into two protein fragments. The expression of this protein is localized to the liver, and peptide fragments of ITIH4 are detected in the serum of patients with liver cancer and cirrhosis. Thus, ITIH4 is a new biomarker for liver disease. Furthermore, experiments using model mice with amyotrophic lateral sclerosis (ALS) have shown that ITIH4 elevation promotes progression of pathological conditions. On the other hand, ITIH4 has many sites that undergo post-translational modification such as glycosylation, and its application to cell staining and flow cytometry by antibodies using peptides and recombinant proteins as antigens has been considered extremely difficult. These problems have been solved by the nanotaxi method, and we present two rat polyclonal antibodies suitable for detection of human ITIH4. | |
| Host | RT | |
| Species specificity | HU RT | |
| Figure 1 | 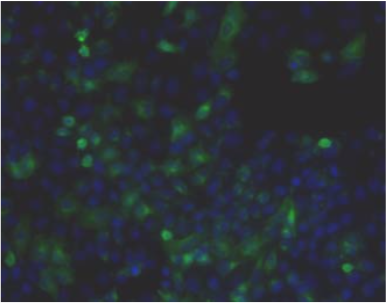 |
|
| Immunohistochemistry. Hela cells ICAFectin441-transfected with a plasmid encoding human ITIH4 were fixed, permeabilized and stained with rat anti-human ITIH4 serum (diluted 1/50) and Alexa488 goat anti rat IgG secondary antibody. Nuclei were counterstained with DAPI. |
||
| Figure 2 | 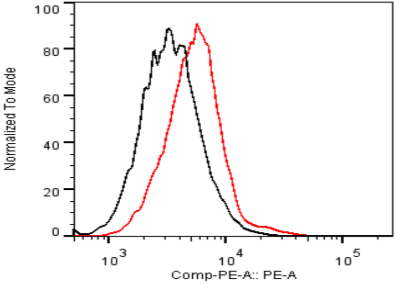 |
|
| Flow cytometry. Untransfected (black) and transfected with ICAfectin 441 and plasmid encoding human ITIH4 (red) Hela cells were fixed, permeabilized and stained with rat anti-human ITIH4 Polyclonal Antibodies diluted 1/100 and R-PE goat anti rat IgG secondary antibody. |
||
| Figure 3 | 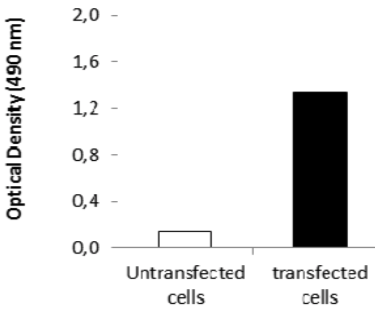 |
|
| ELISA. Lysates of HeLa cells untransfected (red) and ICAFectin441-transfected with a plasmid encoding human ITIH4 were coated in 96 wells plate. Wells were stained with rat anti-human ITIH4 serum and HRP goat anti rat IgG secondary antibody. |
||
| Product name | Anti Inter-Alpha-Trypsin Inhibitor Heavy Chain H4 (ITIH4) pAb (Sprague Dawley Rat, Antiserum) | |
| Cat No | CAC-ICA-TG2-RTP2 | |
| Description | ITIH4 is secreted into the blood and is cleaved by plasma kallikrein into two protein fragments. The expression of this protein is localized to the liver, and peptide fragments of ITIH4 are detected in the serum of patients with liver cancer and cirrhosis. Thus, ITIH4 is a new biomarker for liver disease. Furthermore, experiments using model mice with amyotrophic lateral sclerosis (ALS) have shown that ITIH4 elevation promotes progression of pathological conditions. On the other hand, ITIH4 has many sites that undergo post-translational modification such as glycosylation, and its application to cell staining and flow cytometry by antibodies using peptides and recombinant proteins as antigens has been considered extremely difficult. These problems have been solved by the nanotaxi method, and we present two rat polyclonal antibodies suitable for detection of human ITIH4. | |
| Host | RT | |
| Species specificity | HU RT | |
| Figure 1 | 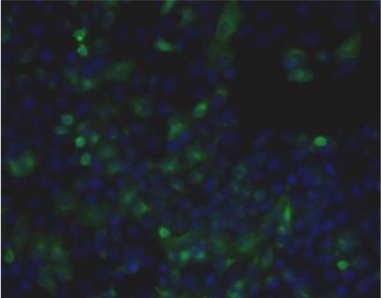 |
|
| Immunohistochemistry. Hela cells ICAFectin441-transfected with a plasmid encoding human ITIH4 were fixed, permeabilized and stained with rat anti-human ITIH4 serum (diluted 1/50) and Alexa488 goat anti rat IgG secondary antibody. Nuclei were counterstained with DAPI. |
||
| Figure 2 | 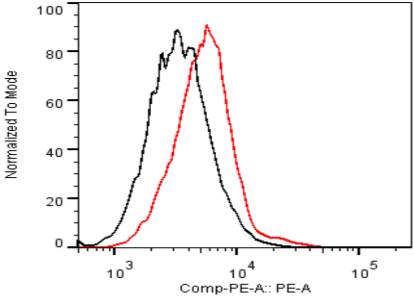 |
|
| Flow Cytometry. HeLa cells untransfected (black) and ICAFectin441-transfected with a plasmid encoding human ITIH4 (red) were stained with rat anti-human ITIH4 serum (diluted 1/100) and R-PE goat anti rat IgG secondary antibody. |
||
| Figure 3 | 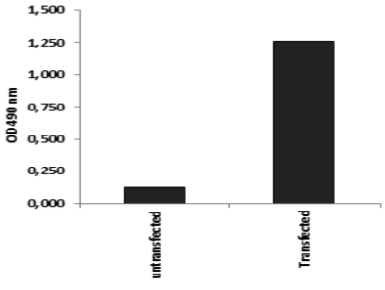 |
|
| ELISA. Lysates of HeLa cells untransfected (red) and ICAFectin441-transfected with a plasmid encoding human ITIH4 were coated in 96 wells plate. Wells were stained with rat anti-human ITIH4 serum and HRP goat anti rat IgG secondary antibody. |
||
| Product name | Anti Human Transmembrane Glycoprotein NMB (GPNMB) pAb (Rabbit, Purified Ig) | |
| Cat No | CAC-ICA-TG1-RBP1 | |
| Description | Lipid-laden macrophages may orchestrate pathology, an accepted notion for inborn lysosomal storage disorders (LSDs) and more recently for metabolic syndrome. The development of enzyme replacement therapy (ERT) for specific LSDs has led in the last decades to the identification of markers of lipid-laden macrophages. In LSDs characterized by foamy macrophages as storage cells, plasma GPNMB has been shown to accurately reflect disease burden. Moreover, GPNMB is also applicable in mouse models of LSDs like Gaucher disease and Niemann-Pick type C. GPNMB is also increased in several acquired diseases, such as metabolic syndrome and neurodegeneration. It therefore might be that these disease conditions share pathophysiological elements, in particular the accumulation of foamy, lysosomal stressed, macrophages. GPNMB is among the most upregulated proteins in lipid-laden macrophages. Nevertheless, at present its exact function in foamy macrophage remains largely enigmatic. Important unanswered questions concern the function(s) served by GPNMB, either the cellular membrane-bound or (extracellular) soluble isoforms, in lipid-laden macrophages and beyond. GPNMB is also expressed in melanocytes. GPNMB has been suggested to be involved in the delay of cell growth and regulation of metastasis. In recent years, Professor Hideaki Hara’s group at Gifu Pharmaceutical University, and others have shown that GPNMB expression is suppressed in mice that have developed amyotrophic lateral sclerosis (ALS), and tracking of GPNMB dynamics and function has been shown to be useful for ALS research. | |
| Host | RAB | |
| Species specificity | HU | |
| Figure 1 | 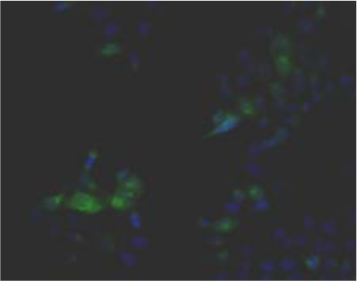 |
|
| Immunohistochemistry. Hela cells ICAFectin441-transfected with a plasmid encoding human GPNMB were fixed, permeabilized and stained with rabbit anti-human GPNMB polyclonal IgG antibody (diluted 1/100) and Alexa488 goat anti rabbit IgG secondary antibody. Nuclei were counterstained with DAPI. |
||
| Figure 2 | 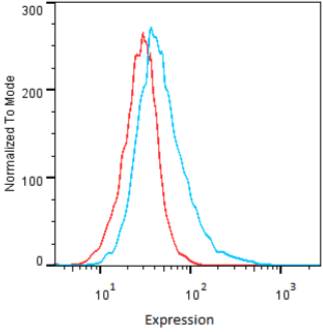 |
|
| Flow cytometry. HeLa cells untransfected (red) and ICAFectin441-transfected with a plasmid encoding human GPNMB (blue) were stained with rabbit anti-human GPNMB polyclonal IgG antibody (diluted 1/100) and R-PE goat anti rabbit IgG secondary antibody. |
||
| Figure 3 | 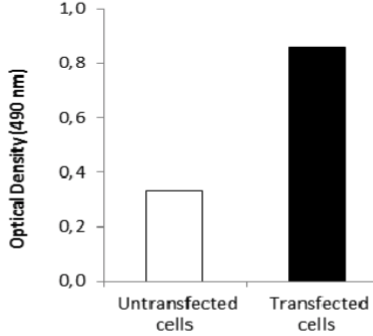 |
|
| ELISA. Lysates of HeLa cells untransfected (red) and ICAFectin441-transfected with a plasmid encoding human GPNMB were coated in 96 wells plate. Wells were stained with anti-human GPNMB polyclonal IgG and HRP goat anti rabbit IgG secondary antibody. |
||
| Product name | Anti Human/Rat Transmembrane Glycoprotein NMB (GPNMB) pAb (Rabbit, Purified Ig) | |
| Cat No | CAC-ICA-TG1-RBP2 | |
| Description | Lipid-laden macrophages may orchestrate pathology, an accepted notion for inborn lysosomal storage disorders (LSDs) and more recently for metabolic syndrome. The development of enzyme replacement therapy (ERT) for specific LSDs has led in the last decades to the identification of markers of lipid-laden macrophages. In LSDs characterized by foamy macrophages as storage cells, plasma GPNMB has been shown to accurately reflect disease burden. Moreover, GPNMB is also applicable in mouse models of LSDs like Gaucher disease and Niemann-Pick type C. GPNMB is also increased in several acquired diseases, such as metabolic syndrome and neurodegeneration. It therefore might be that these disease conditions share pathophysiological elements, in particular the accumulation of foamy, lysosomal stressed, macrophages. GPNMB is among the most upregulated proteins in lipid-laden macrophages. Nevertheless, at present its exact function in foamy macrophage remains largely enigmatic. Important unanswered questions concern the function(s) served by GPNMB, either the cellular membrane-bound or (extracellular) soluble isoforms, in lipid-laden macrophages and beyond. GPNMB is also expressed in melanocytes. GPNMB has been suggested to be involved in the delay of cell growth and regulation of metastasis. In recent years, Professor Hideaki Hara’s group at Gifu Pharmaceutical University, and others have shown that GPNMB expression is suppressed in mice that have developed amyotrophic lateral sclerosis (ALS), and tracking of GPNMB dynamics and function has been shown to be useful for ALS research. | |
| Host | RAB | |
| Species specificity | HU RT | |
| Figure 1 | 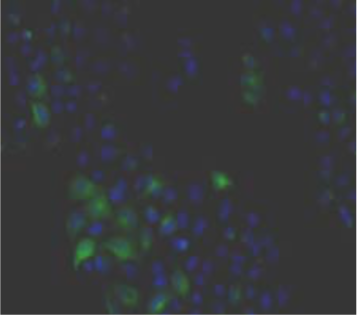 |
|
| Immunohistochemistry. Hela cells ICAFectin441-transfected with a plasmid encoding human GPNMB were fixed, permeabilized and stained with rabbit anti-human GPNMB polyclonal IgG antibody (diluted 1/100) and Alexa488 goat anti rabbit IgG secondary antibody. Nuclei were counterstained with DAPI. |
||
| Figure 2 | 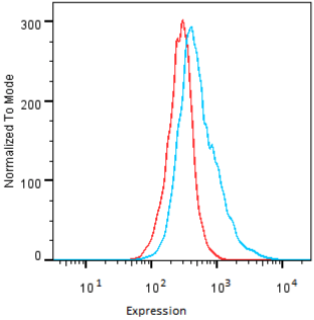 |
|
| Flow cytometry. HeLa cells untransfected (red) and ICAFectin441-transfected with a plasmid encoding human GPNMB (blue) were stained with rabbit anti-human GPNMB polyclonal IgG antibody (diluted 1/100) and R-PE goat anti rabbit IgG secondary antibody. |
||
| Figure 3 | 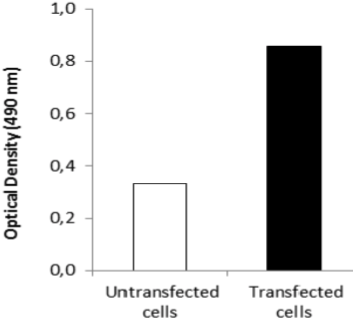 |
|
| ELISA. Lysates of HeLa cells untransfected (red) and ICAFectin441-transfected with a plasmid encoding human GPNMB were coated in 96 wells plate. Wells were stained with anti-human GPNMB polyclonal IgG and HRP goat anti rabbit IgG secondary antibody. |
||
| Product name | Anti Human/Rat/Mouse Transmembrane Glycoprotein NMB (GPNMB) pAb (Rabbit, Purified Ig) | |
| Cat No | CAC-ICA-TG1-RBP3 | |
| Description | Lipid-laden macrophages may orchestrate pathology, an accepted notion for inborn lysosomal storage disorders (LSDs) and more recently for metabolic syndrome. The development of enzyme replacement therapy (ERT) for specific LSDs has led in the last decades to the identification of markers of lipid-laden macrophages. In LSDs characterized by foamy macrophages as storage cells, plasma GPNMB has been shown to accurately reflect disease burden. Moreover, GPNMB is also applicable in mouse models of LSDs like Gaucher disease and Niemann-Pick type C. GPNMB is also increased in several acquired diseases, such as metabolic syndrome and neurodegeneration. It therefore might be that these disease conditions share pathophysiological elements, in particular the accumulation of foamy, lysosomal stressed, macrophages. GPNMB is among the most upregulated proteins in lipid-laden macrophages. Nevertheless, at present its exact function in foamy macrophage remains largely enigmatic. Important unanswered questions concern the function(s) served by GPNMB, either the cellular membrane-bound or (extracellular) soluble isoforms, in lipid-laden macrophages and beyond. GPNMB is also expressed in melanocytes. GPNMB has been suggested to be involved in the delay of cell growth and regulation of metastasis. In recent years, Professor Hideaki Hara’s group at Gifu Pharmaceutical University, and others have shown that GPNMB expression is suppressed in mice that have developed amyotrophic lateral sclerosis (ALS), and tracking of GPNMB dynamics and function has been shown to be useful for ALS research. | |
| Host | RAB | |
| Species specificity | HU MS RT | |
| Figure 1 | 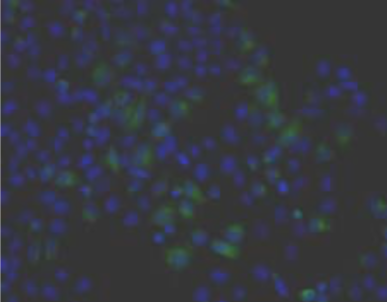 |
|
| Immunohistochemistry. Hela cells ICAFectin441-transfected with a plasmid encoding human GPNMB were fixed, permeabilized and stained with rabbit anti-human GPNMB polyclonal IgG antibody (diluted 1/100) and Alexa488 goat anti rabbit IgG secondary antibody. Nuclei were counterstained with DAPI. |
||
| Figure 2 | 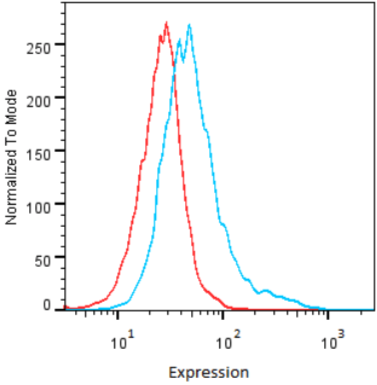 |
|
| Flow cytometry. HeLa cells untransfected (red) and ICAFectin441-transfected with a plasmid encoding human GPNMB (blue) were stained with rabbit anti-human GPNMB polyclonal IgG antibody (diluted 1/100) and R-PE goat anti rabbit IgG secondary antibody. |
||
| Figure 3 | 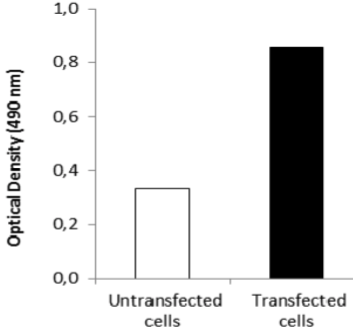 |
|
| ELISA. Lysates of HeLa cells untransfected (red) and ICAFectin441-transfected with a plasmid encoding human GPNMB were coated in 96 wells plate. Wells were stained with anti-human GPNMB polyclonal IgG and HRP goat anti rabbit IgG secondary antibody. |
||
| Product name | Anti WD Repeat Domain Phosphoinositide-Interacting Protein 3 (WIPI-3/WDR45L) pAb (Mouse, Antiserum) | |
| Cat No | CAC-ICA-TG3-MSP1 | |
| Description | WDR45L is a ubiquitously expressed WD40 repeat protein upregulated in a variety of tumor tissues including ovarian and uterine cancers. WDR45L, also known as WIPI-3, comprises seven 40 amino acid (WD40) repeats. WD40 repeats are found in many eukaryotic proteins that coordinate multi-protein complex assemblies. WD40 proteins are implicated in multiple functions, including adaptor/regulatory modules in signal transduction, pre-mRNA processing and cytoskeleton assembly. This protein regulates cell cycle transcription and signal transduction, and it is believed to be involved in cell death by apoptosis and autophagy. In recent years, it has also been used as a marker for neurodegenerative diseases associated with cell death. | |
| Host | MS | |
| Species specificity | MS | |
| Figure 1 | 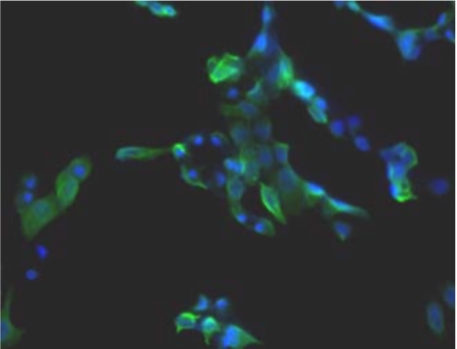 |
|
| Immunohistochemistry. Hela cells ICAFectin441-transfected with a plasmid encoding mouse WDR45L were fixed, permeabilized and stained with rat anti-human ITIH4 serum (diluted 1/100) and Alexa488 goat anti mouse IgG secondary antibody. Nuclei were counterstained with DAPI. |
||
| Figure 2 | 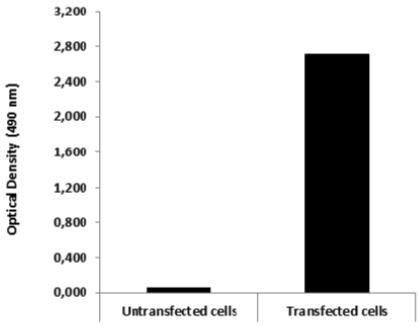 |
|
| ELISA. Lysates of HeLa cells untransfected and ICAFectin441-transfected with a plasmid encoding mouse WDR45L were coated in 96 wells plate. Wells were stained with mouse anti-mouse WDR45L serum and HRP goat anti rat IgG secondary antibody. |
||
| Product name | Anti Cyclin-Dependent Kinase 5 Activator 1, p35 - phospho Ser8 pAb (Rabbit, Affinity Purified) | |
| Cat No | CAC-SDT-02-P35 | |
| Description | Cyclin-dependent kinase 5 (Cdk5) is a unique member of the Cdk family. Its activity in postmitotic neurons is completely dependent upon association with one of two neuronal specific activators, p35 or p39. Cdk5/p35 is involved in a panoply of processes critical to central nervous system function both during development and throughout maturity including neuronal migration during corticogenesis, neurite outgrowth, regulation of the synaptic vesicle cycle, neurotransmitter release, and postsynaptic neurotransmitter receptor regulation and signaling. The mechanisms by which Cdk5 activity is normally regulated remains to be fully delineated. Furthermore, because aberrant Cdk5 activity has been implicated in the etiology of neurodegenerative diseases, identifying the biochemical mechanisms contributing to deregulation of Cdk5 is of substantial biomedical relevance. Deregulation of Cdk5 results from removal of the first 98 amino acids of p35 by the Ca2-dependent cysteine protease, calpain, leaving Cdk5 associated with the N-terminal truncated form p25. Cleavage of p35 to p25 changes the subcellular distribution of active Cdk5 from membranes to the cytosolic fraction, thereby altering substrate specificity. p25 accumulates in neurons undergoing various types of cell death. Expression of Cdk5/p25 in cultured cells results in increased phospho-Tau levels in comparison to cells expressing Cdk5/p35. Furthermore, exogenous overexpression of p25 in transgenic mice results in a neurodegenerative phenotype including the formation of paired helical filaments, Tau aggregation, and neuronal loss similar to that observed in Alzheimer disease. Ca2-dependent activation of the cytoplasmic protease calpain is involved in apoptotic and necrotic cell death. Calpain generally recognizes motifs between conformational domains and cleaves substrate proteins in a limited manner, although the physiological function of calpain activity remains unclear. In some cases calpain cleavage is suspected to be a signaling process. Calpain-mediated cleavage of many proteins including neurofilament proteins, alpha II-spectrin, NR2 subunits of N-methyl-D-aspartic acid receptors, and ezrin is suppressed through phosphorylation. However, how such signaling works and the mechanisms of phosphorylation-dependent inhibition are unknown. Previously we demonstrated that Cdk5 phosphorylated p35, that p35 occurred as a phosphoprotein in neurons, and that the phosphorylation state of p35 affected its susceptibility to calpain cleavage. Phospho-p35 predominates in fetal rat brain and is resistant to the cleavage by calpain, whereas unphosphorylated p35 present during adulthood is more vulnerable to calpain-dependent cleavage. Here we report that Ser8 and Thr138 of p35 serve as the sites of Cdk5-dependent phosphorylation. Furthermore, phosphorylation at these sites reduces the susceptibility of p35 to calpain cleavage. Moreover, specific dephosphorylation of Thr138 increases the susceptibility of p35 to cleavage by calpain in adult rat brains, suggesting that phosphorylation of this site is a particularly critical determinant of Cdk5-dependent neuronal cell death in neurodegenerative diseases. [from: Kamei, H., Saito, T., Ozawa, M., Fujita, Y., Asada, A., Bibb, J.A., Saido, T.C., Sorimachi, H. and Hisanaga, S. Suppression of Calpain-dependent Cleavage of the CDK5 Activator p35 to p25 by Site-specific Phosphorylation J. Biol. Chem. 282(3): 1687-1694 (2007)] |
|
| Host | RAB | |
| Species specificity | HU MS RT | |
| Product name | Anti Septin-5 - phospho Ser17 pAb (Rabbit, Affinity Purified) | |
| Cat No | CAC-SDT-02-SP5 | |
| Description | Septin 5 (Sept5) is a member of the Septin GTPase family and is thought to be involved in exocytosis through interactions with syntaxin 1 in postmitotic neurons. In rats, Sept5 is alternatively spliced to produce a short (Sept5_v2) and long (Sept5_v1) isoform. We recently identified Sept5 in rat brain as a substrate for Cdk5/p35, which phosphorylates Ser17 of Sept5_v1. To date, however, only the short Sept5_v2 isoform has been reported in the mouse. To determine the general expression of the Sept5_v1 isoform in mammals, we isolated Sept5_v1 cDNA by PCR using mouse brain total RNA. Mouse Sept5_v1 cDNA showed a high degree of nucleotide and amino acid sequence homology to the corresponding isoform of rat and human Sept5. Both isoforms were expressed mainly in brain and testis at the mRNA level, but expression was restricted to brain at the protein level. Whereas Sept5_v1 mRNA was highly expressed in the cortex and hippocampus, Sept5_v2 mRNA was expressed at the similar extent across in various brain regions. The protein ratio of Sept5_v1 to Sept5_v2 was high in the hippocampus, roughly equivalent in the cortex and low in the cerebellum and medulla. Sept5_v2 expression increased gradually from E17 to P30, but expression of Sept5_v1 was delayed until P10. The two isoforms were distinguished by their pattern of N-terminal phosphorylation. Thus, these different expression and phosphorylation patterns suggest isoform-specific functions for Sept5 and that a phosphorylation-specific antibody will be useful to study this idea. [from: Asada A1, Takahashi J, Taniguchi M, Yamamoto H, Kimura T, Saito T, Hisanaga S. Neuronal expression of two isoforms of mouse Septin 5. J Neurosci Res. 2010 May 1;88(6):1309-16. doi: 10.1002/jnr.22294.] | |
| Host | RAB | |
| Species specificity | MS | |
| Product name | Anti 1-Oleoyl-Palmitoyl-Phosphatidylcholine (OPPC) mAb (Clone 15-3C1) | |
| Cat No | CAC-SK-KC01-M01 | |
| Description | In neurons, the plasma membrane is functionally separated into several distinct segments. Neurons form these domains by delivering selected components to and by confining them within each segment of the membrane. Although some mechanisms of the delivery are elucidated, that of the confinement is unclear.We show here that 1-oleoyl-2-palmitoyl-phosphatidylcholine (OPPC),a unique molecular species of phospholipids, is concentrated at the protrusion tips of several neuronal culture cells and the presynaptic area of neuronal synapses of the mouse brain. InPC12 cells, NGF-stimulated neuronal differentiation induces a phospholipase A1 activity at the protrusion tips, which co-localizes with the OPPC domain. Inhibition of the phospholipase A1activity leads to suppression of phospholipid remodeling in the tip membrane and results in disappearance of the OPPC at the tips. In these cells, confinement of dopamine transporter and G0 proteins to the tip was also disrupted. These findings link the lateral distribution of the molecular species of phospholipids to the formation of functional segments in the plasma membrane of neurons and to the mechanism of protein confinement at the synapse. The Cosmobio Antibody Collection (CAC) has registered a monoclonal antibody (clone 15-3C1) that specifically recognizes the phospholipid 1-Oleoyl-Palmitoyl-Phosphatidylcholine (OPPC) localized at neurite tips. Recently, Kuge et al found that OPPC controls protein localization (dopamine transport protein and G protein). This suggests that phospholipid remodeling is performed in the cell membrane, and that OPPC may play an important role in neurotransmission and be related to pathologies such as Parkinson's disease and dementia. Antibody Source: Professor Koichi Honke of Kochi University School of Medicine. [from: Kuge, H., Akahori, K., Yagyu K., Honke K. Functional Compartmentalization of the Plasma Membrane of Neurons by a Unique Acyl Chain Composition of Phospholipids The Journal Of Biological Chemistry (2014) 289(39): 26783–26793.] |
|
| Host | MS | |
| Species specificity | - | |
| Figure 1 | 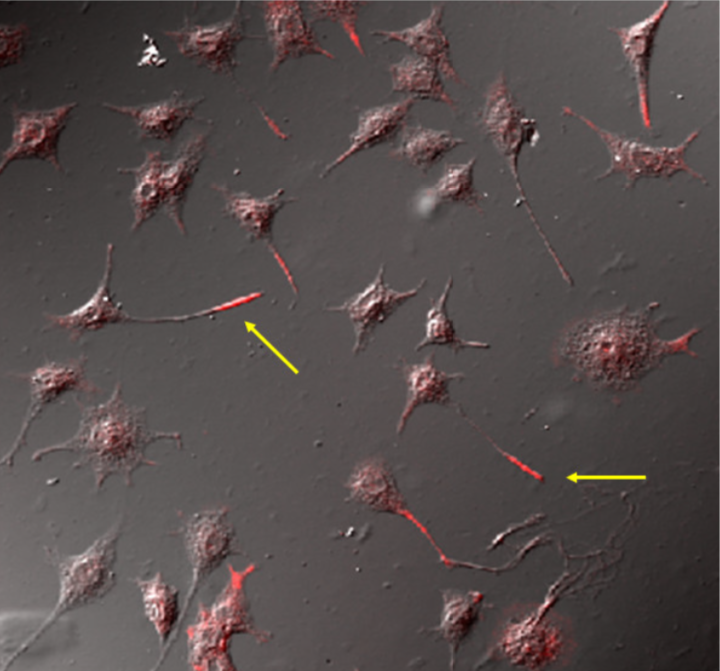 |
|
| Immunolocalization of phospholipid OPPC to the neurite tip (red) in cultured rat PC12 neurons using mAb clone 15-3C1. | ||
| Muscarinic acetylcholine receptor antibodies (Top) | ||
| Muscarinic acetylcholine receptors, or mAChRs, are acetylcholine receptors that form G protein-coupled receptor complexes in the cell membranes of certain neurons and other cells. They play several roles, including acting as the main end-receptor stimulated by acetylcholine released from postganglionic fibers in the parasympathetic nervous system. Muscarinic receptors are so named because they are more sensitive to muscarine than to nicotine. By the use of selective radioactively labeled agonist and antagonist substances, five subtypes of muscarinic receptors have been determined, named M1-M5 (using an upper case M and subscript number). M1, M3, M5 receptors are coupled with Gq proteins, while M2 and M4 receptors are coupled with Gi/o proteins. | ||
| Product name | Anti Muscarinic Acetylcholine Receptor M1(CHRM1) pAb (Rabbit, Antiserum) | |
| Cat No | CAC-YCU-PS-M1 | |
| Description | M1 receptor: This receptor is found mediating slow EPSP at the ganglion in the postganglionic nerve[citation needed], is common in exocrine glands and in the CNS. It is predominantly found bound to G proteins of class Gq, which use upregulation of phospholipase C and, therefore, inositol trisphosphate and intracellular calcium as a signaling pathway. A receptor so bound would not be susceptible to CTX or PTX. However, Gi (causing a downstream decrease in cAMP) and Gs (causing an increase in cAMP) have also been shown to be involved in interactions in certain tissues, and so would be susceptible to PTX and CTX, respectively. References: 1) K. Shiozaki, E. Iseki, H. Uchiyama, Y. Watanabe, T. Haga, K. Kameyama, T. Yamada, T. Yamamoto, K. Kosaka Alterations of muscarinic acetylcholine receptor subtypes in diffuse Lewy body disease: relation to Alzheimer's's disease J Neurol Neurosurg Psychiatry. 1999 Aug; 67 (2): 209-13. 2) Kazumasa Shiozaki, Eizo Iseki, Hiroaki Hino, Kenji Kosaka Distribution of m1 acetylcholine receptors in the hippocampus of patients with patients and patients with dementia with Lewy bodies-an immunohistochemistry study J Neurol Sci. 2001 Dec 15. |
|
| Host | RAB | |
| Species specificity | HU POR | |
| Product name | Anti Muscarinic Acetylcholine Receptor M2 (CHRM2) pAb (Rabbit, Antiserum) | |
| Cat No | CAC-YCU-PS-M2 | |
| Description | M2 receptor: The M2 muscarinic receptors are located in the heart, where they act to slow the heart rate down to normal sinus rhythm, by slowing the speed of depolarization. In humans under resting conditions vagal activity dominates over sympathetic activity. Hence inhibition of m2 receptors (e.g. by atropine) will cause a raise in heart rate. They also moderately reduce contractile forces of the atrial cardiac muscle, and reduce conduction velocity of the atrioventricular node (AV node). It also serves to slightly decrease the contractile forces of the ventricular muscle. References: 1) K. Shiozaki, E. Iseki, H. Uchiyama, Y. Watanabe, T. Haga, K. Kameyama, T. Yamada, T. Yamamoto, K. Kosaka Alterations of muscarinic acetylcholine receptor subtypes in diffuse Lewy body disease: relation to Alzheimer's's disease J Neurol Neurosurg Psychiatry. 1999 Aug; 67 (2): 209-13. 2) Kazumasa Shiozaki, Eizo Iseki, Hiroaki Hino, Kenji Kosaka Distribution of m1 acetylcholine receptors in the hippocampus of patients with patients and patients with dementia with Lewy bodies-an immunohistochemistry study J Neurol Sci. 2001 Dec 15. |
|
| Host | RAB | |
| Species specificity | HU POR | |
| Product name | Anti Muscarinic Acetylcholine Receptor M3 (CHRM3) pAb (Rabbit, Antiserum) | |
| Cat No | CAC-YCU-PS-M3 | |
| Description | M3 receptor: The M3 muscarinic receptors are located at many places in the body. They are located in the smooth muscles of the blood vessels, as well as in the lungs. Because the M3 receptor is Gq-coupled and mediates an increase in intracellular calcium, it typically causes contraction of smooth muscle, such as that observed during bronchoconstriction and bladder voiding. However, with respect to vasculature, activation of M3 on vascular endothelial cells causes increased synthesis of nitric oxide, which diffuses to adjacent vascular smooth muscle cells and causes their relaxation, thereby explaining the paradoxical effect of parasympathomimetics on vascular tone and bronchiolar tone. Indeed, direct stimulation of vascular smooth muscle, M3 mediates vasconstriction in pathologies wherein the vascular endothelium is disrupted. The M3 receptors are also located in many glands, which help to stimulate secretion in, for example, the salivary glands, as well as other glands of the body. References: 1) K. Shiozaki, E. Iseki, H. Uchiyama, Y. Watanabe, T. Haga, K. Kameyama, T. Yamada, T. Yamamoto, K. Kosaka Alterations of muscarinic acetylcholine receptor subtypes in diffuse Lewy body disease: relation to Alzheimer's's disease J Neurol Neurosurg Psychiatry. 1999 Aug; 67 (2): 209-13. 2) Kazumasa Shiozaki, Eizo Iseki, Hiroaki Hino, Kenji Kosaka Distribution of m1 acetylcholine receptors in the hippocampus of patients with patients and patients with dementia with Lewy bodies-an immunohistochemistry study J Neurol Sci. 2001 Dec 15. |
|
| Host | RAB | |
| Species specificity | HU RT | |
| Product name | Anti Muscarinic Acetylcholine Receptor M4 (CHRM4) pAb (Rabbit, Antiserum) | |
| Cat No | CAC-YCU-PS-M4 | |
| Description | M4 muscarinic receptors are coupled to Gi/o heterotrimeric proteins. They function as inhibitory autoreceptors for acetylcholine. Activation of M4 receptors inhibits acetylcholine release in the striatum. The M2 subtype of acetylcholine receptor functions similarly as an inhibitory autoreceptor to acetylcholine release, albeit functioning actively primarily in the hippocampus and cerebral cortex. Muscarinic acetylcholine receptors possess a regulatory effect on dopaminergic neurotransmission. Activation of M4 receptors in the striatum inhibit D1-induced locomotor stimulation in mice. M4 receptor-deficient mice exhibit increased locomotor simulation in response to D1 agonists, amphetamine and cocaine. Neurotransmission in the striatum influences extrapyramidal motor control, thus alterations in M4 activity may contribute to conditions such as Parkinson's Disease. [Muscarinic acetylcholine receptor M4, https://en.wikipedia.org/w/index.php?title=Muscarinic_acetylcholine_receptor_M4&oldid=914772257 (last visited Oct. 1, 2019).] References: 1) K. Shiozaki, E. Iseki, H. Uchiyama, Y. Watanabe, T. Haga, K. Kameyama, T. Yamada, T. Yamamoto, K. Kosaka Alterations of muscarinic acetylcholine receptor subtypes in diffuse Lewy body disease: relation to Alzheimer's's disease J Neurol Neurosurg Psychiatry. 1999 Aug; 67 (2): 209-13. 2) Kazumasa Shiozaki, Eizo Iseki, Hiroaki Hino, Kenji Kosaka Distribution of m1 acetylcholine receptors in the hippocampus of patients with patients and patients with dementia with Lewy bodies-an immunohistochemistry study J Neurol Sci. 2001 Dec 15. |
|
| Host | RAB | |
| Species specificity | HU RT | |
| Miscellaneous (Top) | ||
| Product name | Anti Calcium/Calmodulin-Dependent Protein Kinase II, Isoform C (dCAMKII) mAb (Clone 18) | |
| Cat No | CAC-TNL-001-CAM | |
| Description | Ca2+/calmodulin-dependent protein kinase II (CaM kinase II or CaMKII) is a serine/threonine-specific protein kinase that is regulated by the Ca2+/calmodulin complex. CaMKII is involved in many signaling cascades and is thought to be an important mediator of learning and memory. CaMKII is also necessary for Ca2+ homeostasis and reuptake in cardiomyocytes,[2] chloride transport in epithelia,[3] positive T-cell selection, and CD8 T-cell activation. Misregulation of CaMKII is linked to Alzheimer’s disease, Angelman syndrome, and heart arrhythmia. [from: Wikipedia contributors. (2019, March 15). Ca2+/calmodulin-dependent protein kinase II. In Wikipedia, The Free Encyclopedia. Retrieved 20:14, June 4, 2019, from https://en.wikipedia.org/w/index.php?title=Ca2%2B/calmodulin-dependent_protein_kinase_II&oldid=887834881] References: Takamatsu Y, Kishimoto Y and Ohsako S. (2003) Immunohistochemical study of Ca2+/calmodulin-dependent protein kinase II in the Drosophila brain using a specific monoclonal antibody. Brain Res. 974:99-116. |
|
| Host | MS | |
| Species specificity | Drosophila | |
| Product name | Anti Schwann Cell/Peripheral Myelin mAb (Clone Schwann/2E) | |
| Cat No | CAC-GU01-M01AS-A | |
| Description | Myelin is a lipid-rich (fatty) substance formed in the central nervous system (CNS) by glial cells called oligodendrocytes, and in the peripheral nervous system (PNS) by Schwann cells. Myelin insulates nerve cell axons to increase the speed at which information (encoded as an electrical signal) travels from one nerve cell body to another (as in the CNS) or, for example, from a nerve cell body to a muscle (as in the PNS). The myelinated axon can be likened to an electrical wire (the axon) with insulating material (myelin) around it. However, unlike the plastic covering on an electrical wire, myelin does not form a single long sheath over the entire length of the axon. Rather, each myelin sheath insulates the axon over a single section and, in general, each axon comprises multiple long myelinated sections separated from each other by short gaps called Nodes of Ranvier. Each myelin sheath is formed by the concentric wrapping of an oligodendrocyte or Schwann cell process around the axon. More precisely, myelin speeds the transmission of electrical impulses called action potentials along myelinated axons by insulating the axon and reducing axonal membrane capacitance. On a molecular level, it increases the distance between the cations on the outside of the axon and the Na⁺ ions that move through the axoplasm during an action potential, thereby greatly reducing the magnitude of the repulsive forces (which are inversely proportional to the square of the distance, as per Coulomb's law) between them that would otherwise act to inhibit the movement of the Na⁺-ions. The discontinuous structure of the myelin sheath results in saltatory conduction whereby the action potential "jumps" from one node of Ranvier, over a long myelinated stretch of the axon called the internode, before "recharging" at the next node of Ranvier, and so on, until it reaches the axon terminal. Nodes of Ranvier are the short (~1 micron) unmyelinated regions of the axon between adjacent long (~0.2 mm - >1 mm) myelinated internodes. Once it reaches the axon terminal, this electrical signal provokes the release of a chemical message or neurotransmitter that binds to receptors on the adjacent post-synaptic cell (e.g. nerve cell in the CNS or muscle cell in the PNS) at specialized regions called synapses. This "insulating" role for myelin is essential for normal motor function (i.e. movement such as walking), sensory function (e.g. hearing, seeing or feeling the sensation of pain) and cognition (e.g. acquiring and recalling knowledge), as demonstrated by the consequences of disorders that affect it, such as the genetically determined leukodystrophies; the acquired inflammatory demyelinating disorder, multiple sclerosis; and the inflammatory demyelinating peripheral neuropathies. Due to its high prevalence, multiple sclerosis, which specifically affects the central nervous system (brain, spinal cord and optic nerve), is the best known disorder of myelin. [from: Wikipedia contributors. (2019, June 2). Myelin. In Wikipedia, The Free Encyclopedia. Retrieved 20:18, June 4, 2019, from https://en.wikipedia.org/w/index.php?title=Myelin&oldid=899981120] References: 1) Nakazato Y, et al.: Monoclonal antibodies which recognize phosphorylated and nonphosphorylated epitopes of neurofilament protein. Biomed Res 1987, 8:369-376. 2) Arai H, et al. (1998) A novel marker of Schwann cells and myelin of the peripheral nervous system. Pathology Int. 48:206-214. 3) 新井華子, 他 (1998) シュワン細胞と末梢性髄鞘の新しいマーカー(Schwann/2E 抗体)によるシュワン細胞由来腫瘍の検索. Kitakanto Med. J. 48:1-8. 4) Jasmin L, et a. (2000) Schwann cells are removed from the spinal cord after effecting recovery from paraplegia. J Neurosci. 20:9215-9223. 5) Jasmin L, et al. (2002) Remyelination within the CNS: do schwann cells pave the way for oligodendrocytes? Neuroscientist. 8:198-203. 6) 舟生勇人, 他(2003) 中頭蓋窩硬膜より発生したintracranial schwannoma の稀な1 例. 脳神経外科. 31:789-793. |
|
| Host | MS | |
| Species specificity | HU MS RT | |
| Figure 1 | 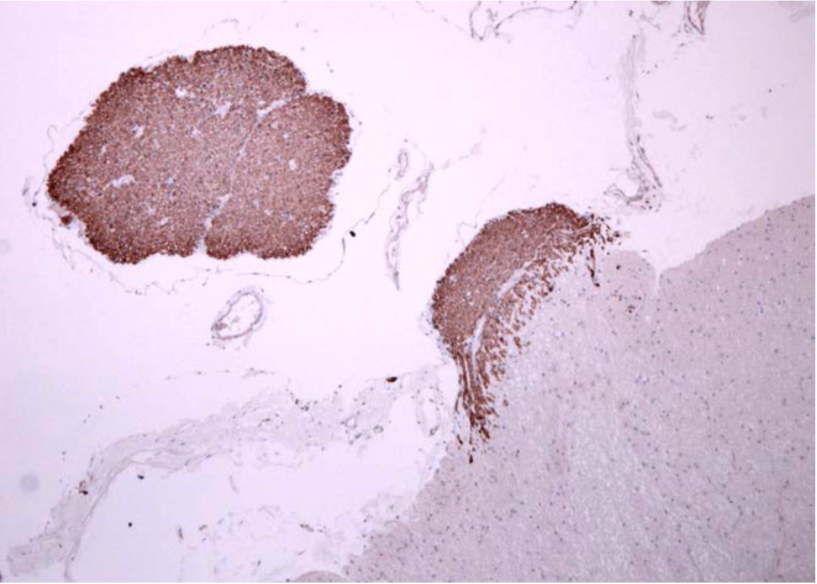 |
|
| Immunohistochemical analysis of Schwann/2E. Schwann/2E positively reacts to spinal nerves but not to parenchyma (lower light area) Sample: Human spinal cord (paraffin embedded/formaldehyde fixed). Dilution: 1/10000. Staining method: Streptavidin/biotin. |
||
| Figure 2 | 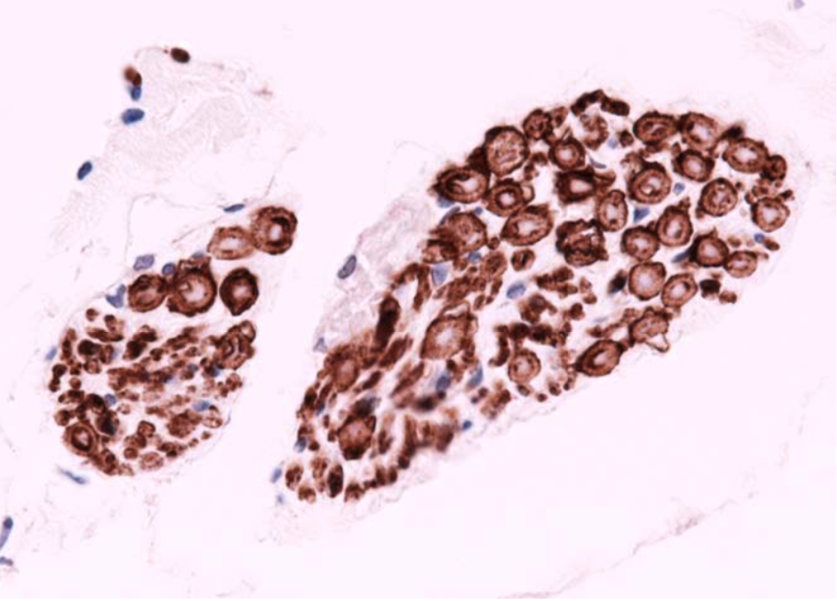 |
|
| Immunostaining of spinal nerves with Schwann/2E. Schwann cells and myelin sheath are positively stained with Schwann/2E. |
||
