 CAC Antibody Collection
CAC Antibody Collection
The antibodies on this page are part of Cosmo Bio's exclusive CAC Collection. For many many thousands of other antibodies from many different makers, use our Search the Store function and our Explore Products drop down menu.
TGF-beta pathway
TGF-beta LAP-d / TGF-beta signalingLatency-Associated Peptide (LAP)
Transforming growth factor-beta (TGF-beta) exists in five isoforms, three of which are expressed in mammals and designated as TGF-beta 1, TGF-beta 2, and TGF-beta 3. The TGF-betas and their receptors are ubiquitously expressed in normal tissue and most cell lines. It then follows that if there is such widespread expression of a ligand and its receptor there must be several levels of regulation of TGF-beta-mediated activity to achieve biological relevance. It is easy to conceptualize regulation of TGF-beta function to occur by virtue of the quantity of TGF-beta produced by induction of transcription and translation of TGF-beta. Alternatively, the expression of known TGF-beta signaling receptors could be differentially expressed to control TGF-beta activity. However, the most important regulation of TGF-beta activity to be reported is based on whether or not TGF-beta is biologically active or latent. This is because TGF-betas are generally secreted in a biologically latent form called either small or large latent-TGF-beta (L-TGF-beta). L-TGF-beta cannot interact with its receptor and is therefore biologically inert. Thus, the mechanism(s) by which L-TGF-beta can be activated by the cell releasing TGF-beta, the target cell of TGF-beta or the cellular milieu would then have the greatest impact on the biological effect of TGF-beta.
The isoforms of TGF-beta are encoded as large precursor proteins that are 390–412 amino acids in size and each isoform is the product of a separate gene. TGF-beta proteins undergo a number of processing steps intracellularly prior to their secretion by a cell. The most important processing step appears to be the proteolytic digestion of the precursor by the endopeptidase furin, which cleaves the TGF-beta protein between amino acids 278 and 279. The proteolysis yields two products that assemble into dimers. The 65–75-kDa dimer protein from the N-terminal region is called the latency-associated peptide (LAP), while a second 25-kDa dimer from the C-terminal portion of the precursor is called the mature TGF-beta. Despite the cleavage of the precursor a common feature of all TGF-betas is that the N-terminal portion remains noncovalently associated with the rest of the protein. The presence of the LAP protein facilitates transit of TGF-beta from the cell and makes the TGF-beta biologically inactive. [from: Khalil N. TGF-β: from latent to active (1999) Microbes and Infection 1(15): 1255-1263.]
The isoforms of TGF-beta are encoded as large precursor proteins that are 390–412 amino acids in size and each isoform is the product of a separate gene. TGF-beta proteins undergo a number of processing steps intracellularly prior to their secretion by a cell. The most important processing step appears to be the proteolytic digestion of the precursor by the endopeptidase furin, which cleaves the TGF-beta protein between amino acids 278 and 279. The proteolysis yields two products that assemble into dimers. The 65–75-kDa dimer protein from the N-terminal region is called the latency-associated peptide (LAP), while a second 25-kDa dimer from the C-terminal portion of the precursor is called the mature TGF-beta. Despite the cleavage of the precursor a common feature of all TGF-betas is that the N-terminal portion remains noncovalently associated with the rest of the protein. The presence of the LAP protein facilitates transit of TGF-beta from the cell and makes the TGF-beta biologically inactive. [from: Khalil N. TGF-β: from latent to active (1999) Microbes and Infection 1(15): 1255-1263.]
| TGF-beta pathway: TGF-beta LAP-d | |||
| Product name (click for order info) | Cat No (click for datasheet) |
Host | Species specificity |
| Anti Latency-Associated Peptide (LAP) Plasma Kallikrein Degradation Fragment L59 pAb (Rabbit, Affinity Purified) | CAC-RIK-CP-PT59 | RAB | HU RT |
| Anti Latent TGF-Beta (Plasmin Degradation Fragment L57) pAb (Rabbit, Affinity Purified) | CAC-RIK-CP-PT57 | RAB | HU |
| Anti Latency-Associated Peptide (LAP) Plasma Kallikrein Degradation Fragment R58 mAb (Clone 18F9-16) | CAC-RIK-MA-R58 | MS | MS |
| Anti Latency-Associated Peptide (LAP) Plasma Kallikrein Degradation Fragment L59 mAb (Clone 6D6) | CAC-RIK-MA-L59 | MS | HU |
| Product name | Anti Latency-Associated Peptide (LAP) Plasma Kallikrein Degradation Fragment L59 pAb (Rabbit, Affinity Purified) |
| Cat No | CAC-RIK-CP-PT59 |
| Description | TGF-β is produced as a pro-protein in which the 25 kD active TGF-β is trapped by an N-terminal pro-peptide called Latency Associated Protein (LAP). Upon receipt of certain stimuli a conformational change is induced in a latent complex to release the active TGF-β from the complex. The resultant TGF-β binds to cognate signaling receptors and exerts various physiological and pathological activities. This reaction is called TGF-β activation reaction, which is known to be induced by binding of the latent complex to cell adhesion proteins such as thrombospondin and integrins, and/or by being cleaved by the action of proteases such as serine proteases, cysteine proteases, and MMPs in an organ and context-depending manner. The RIKEN Center for Biomedical Science and Research Center for Liver Cancer Prevention and Research Unit focused on the involvement of the serine protease plasmin and plasma kallikrein in the release and activation of TGF-β and its involvement in liver diseases. They showed that plasmin and plasma kallikrein cleave, respectively, at 56Lys-57Leu and 58Arg-59Leu within the LAP portion of the latent TGF-β1 molecule. The anti-TGF-β1 LAP-degradates (LAP-D) antibodies are useful to investigate the molecular mechanism of TGF-β activation and its related diseases including liver fibrosis/cirrhosis and liver degeneration. Source: Professor Koichi, National Institute of Advanced Industrial Science and Technology RIKEN Biomedical Science Research Center Liver Cancer Prevention Research Unit. References: Kojima, S. et al., "Detection and prevention of hepatic fibrosis targetingTGF-β activation reaction" Hepatology 46(4): 712A (2007). |
| Host | Rabbit |
| Species specificity | HU RT |
| Figure 1 | 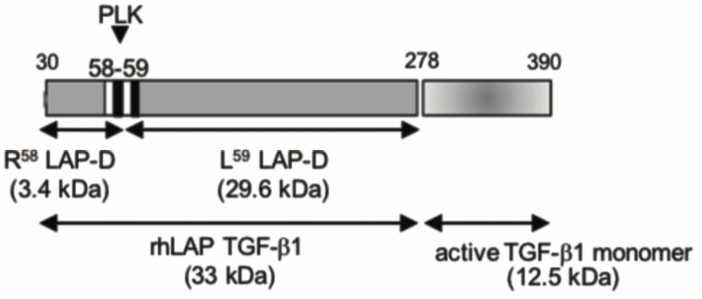 |
| Model depicting the N-terminal cut end of LAP-degradate (LAP-D) L59 when latent TGF-β is digested with Plasma Kallikrein (PLK). | |
| Figure 2 | 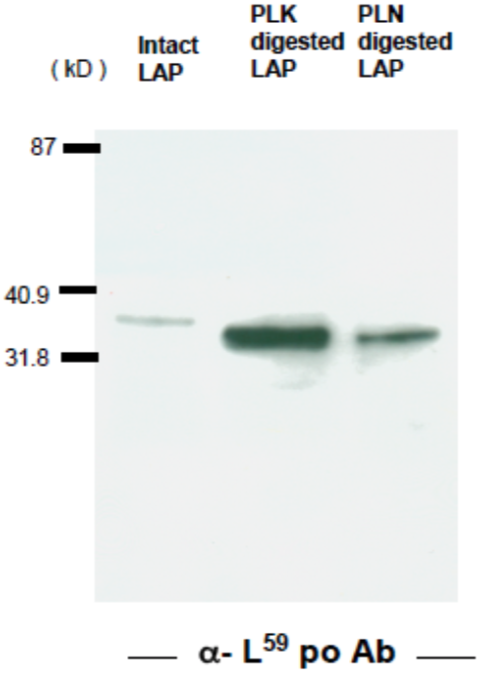 |
| Immunoblot analysis of GST-TGFβ after cleavage with Plasma Kallikrein. Anti-latent TGF-beta (LAP-degradate L59) pAb recognizes the N-terminal cut end of LAP-degradate K59, released when latent TGF-β is digested with Plasma Kallikrein (PLK). | |
| Product name | Anti Latent TGF-Beta (Plasmin Degradation Fragment L57) pAb (Rabbit, Affinity Purified) |
| Cat No | CAC-RIK-CP-PT57 |
| Description | TGF-β is produced as a pro-protein in which the 25 kD active TGF-β is trapped by an N-terminal pro-peptide called Latency Associated Protein (LAP). Upon receipt of certain stimuli a conformational change is induced in a latent complex to release the active TGF-β from the complex. The resultant TGF-β binds to cognate signaling receptors and exerts various physiological and pathological activities. This reaction is called TGF-β activation reaction, which is known to be induced by binding of the latent complex to cell adhesion proteins such as thrombospondin and integrins, and/or by being cleaved by the action of proteases such as serine proteases, cysteine proteases, and MMPs in an organ and context-depending manner. The RIKEN Center for Biomedical Science and Research Center for Liver Cancer Prevention and Research Unit focused on the involvement of the serine protease plasmin and plasma kallikrein in the release and activation of TGF-β and its involvement in liver diseases. They showed that plasmin and plasma kallikrein cleave, respectively, at 56Lys-57Leu and 58Arg-59Leu within the LAP portion of the latent TGF-β1 molecule. The anti-TGF-β1 LAP-degradates (LAP-D) antibodies are useful to investigate the molecular mechanism of TGF-β activation and its related diseases including liver fibrosis/cirrhosis and liver degeneration. Source: Professor Koichi, National Institute of Advanced Industrial Science and Technology RIKEN Biomedical Science Research Center Liver Cancer Prevention Research Unit. References: Kojima, S. et al., "Detection and prevention of hepatic fibrosis targetingTGF-β activation reaction" Hepatology 46(4): 712A (2007). |
| Host | Rabbit |
| Species specificity | HU |
| Figure 1 | 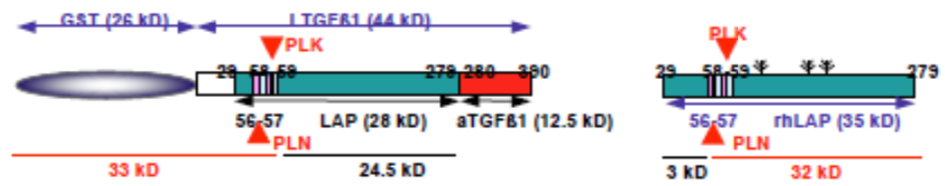 |
| Model depicting the N-terminal cut end of LAP degradate (LAP-D) L57 when latent TGF-β is digested with Plasmin (PLN). | |
| Figure 2 | 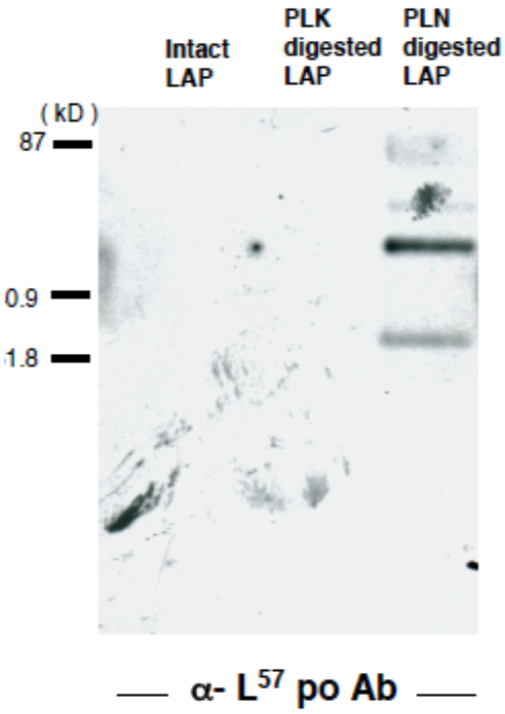 |
| Immunoblot analysis of GST-TGFβ after cleavage with Plasmin. Anti Latent TGF-BETA(LAP-degradate L57) pAb recognizes the N-terminal cut end of LAP-degradate L57, released when latent TGF-β is digested with Plasmin (PLN). | |
| Product name | Anti Latency-Associated Peptide (LAP) Plasma Kallikrein Degradation Fragment R58 mAb (Clone 18F9-16) |
| Cat No | CAC-RIK-MA-R58 |
| Description | TGF-β is produced as a pro-protein in which the 25 kD active TGF-β is trapped by an N-terminal pro-peptide called Latency Associated Protein (LAP). Upon receipt of certain stimuli a conformational change is induced in a latent complex to release the active TGF-β from the complex. The resultant TGF-β binds to cognate signaling receptors and exerts various physiological and pathological activities. This reaction is called TGF-β activation reaction, which is known to be induced by binding of the latent complex to cell adhesion proteins such as thrombospondin and integrins, and/or by being cleaved by the action of proteases such as serine proteases, cysteine proteases, and MMPs in an organ and context-depending manner. The RIKEN Center for Biomedical Science and Research Center for Liver Cancer Prevention and Research Unit focused on the involvement of the serine protease plasmin and plasma kallikrein in the release and activation of TGF-β and its involvement in liver diseases. They showed that plasmin and plasma kallikrein cleave, respectively, at 56Lys-57Leu and 58Arg-59Leu within the LAP portion of the latent TGF-β1 molecule. The anti-TGF-β1 LAP-degradates (LAP-D) antibodies are useful to investigate the molecular mechanism of TGF-β activation and its related diseases including liver fibrosis/cirrhosis and liver degeneration. Source: Professor Koichi, National Institute of Advanced Industrial Science and Technology RIKEN Biomedical Science Research Center Liver Cancer Prevention Research Unit. References: Hara M., Kirita A., Kondo W. et al. "LAP degradation product reflects plasma kallikrein-dependent TGF-β activation in patients with hepatic fibrosis" Springerplus. May 1; 3: 221. PMCID: PMC4033717 (2014). |
| Host | Mouse |
| Species specificity | MS |
| Figure 1 | 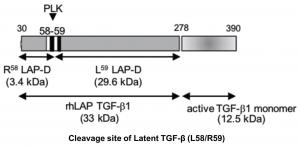 |
| Model depicting the C-terminal cut end of LAP-degradate (LAP-D) R58 when latent TGF-β is digested with Plasma Kallikrein (PLK). | |
| Figure 2 | 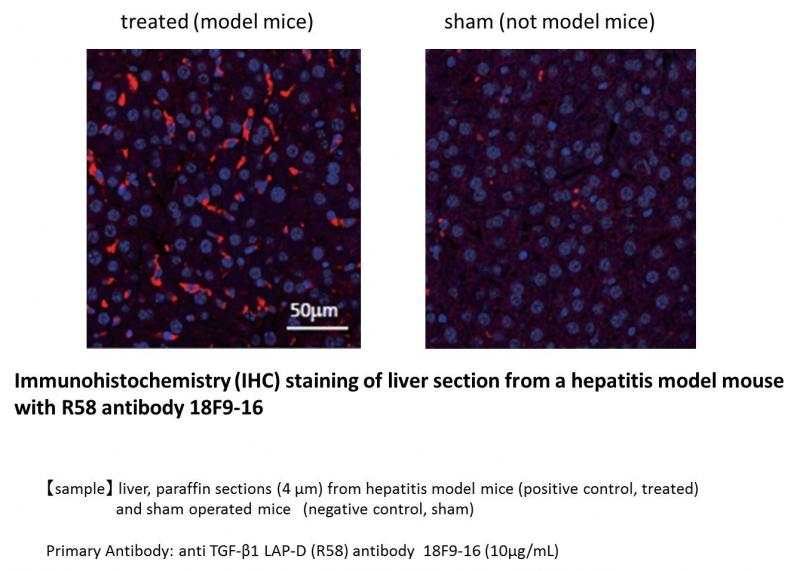 |
| Immunohistochemical (IHC) analysis of liver sections from hepatitis model mice with anti TGF-β1 LAP-D (R58) antibody (10μg/mL). Liver paraffin sections (4 μm) from hepatitis model mice (positive control, treated) and sham operated mice (negative control, sham). | |
| Product name | Anti Latency-Associated Peptide (LAP) Plasma Kallikrein Degradation Fragment L59 mAb (Clone 6D6) |
| Cat No | CAC-RIK-MA-L59 |
| Description | TGF-β is produced as a pro-protein in which the 25 kD active TGF-β is trapped by an N-terminal pro-peptide called Latency Associated Protein (LAP). Upon receipt of certain stimuli a conformational change is induced in a latent complex to release the active TGF-β from the complex. The resultant TGF-β binds to cognate signaling receptors and exerts various physiological and pathological activities. This reaction is called TGF-β activation reaction, which is known to be induced by binding of the latent complex to cell adhesion proteins such as thrombospondin and integrins, and/or by being cleaved by the action of proteases such as serine proteases, cysteine proteases, and MMPs in an organ and context-depending manner. The RIKEN Center for Biomedical Science and Research Center for Liver Cancer Prevention and Research Unit focused on the involvement of the serine protease plasmin and plasma kallikrein in the release and activation of TGF-β and its involvement in liver diseases. They showed that plasmin and plasma kallikrein cleave, respectively, at 56Lys-57Leu and 58Arg-59Leu within the LAP portion of the latent TGF-β1 molecule. The anti-TGF-β1 LAP-degradates (LAP-D) antibodies are useful to investigate the molecular mechanism of TGF-β activation and its related diseases including liver fibrosis/cirrhosis and liver degeneration. Source: Professor Koichi, National Institute of Advanced Industrial Science and Technology RIKEN Biomedical Science Research Center Liver Cancer Prevention Research Unit. References: 1) LAP degradation product reflects plasma kallikrein-dependent TGF-β activation in patients with hepatic fibrosis, Hara M., Kirita A., Kondo W. et al. Springerplus. May 1; 3: 221. PMCID: PMC4033717 (2014). 2) L59 TGF-β LAP degradation products serve as a promising blood biomarker for liver fibrogenesis in mice, Hara M., Inoue I., Yamazaki Y. et al. Fibrogenesis Tissue Repair. Sep 15; 8: 17. PMCID: PMC4570586 (2015). |
| Host | Mouse |
| Species specificity | HU |
| Figure 1 | 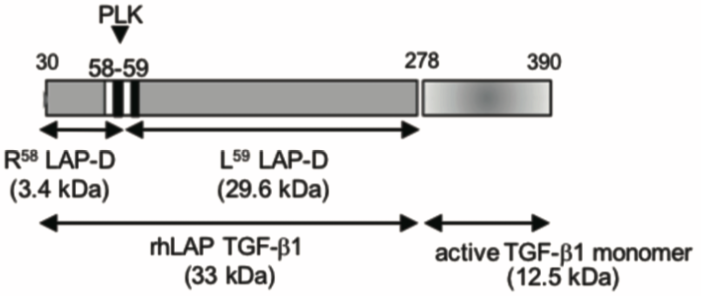 |
| Model depicting the N-terminal cut end of LAP degradate (LAP-D) L59 when latent TGF-β is digested with Plasma Kallikrein (PLK). | |
| Figure 2 | 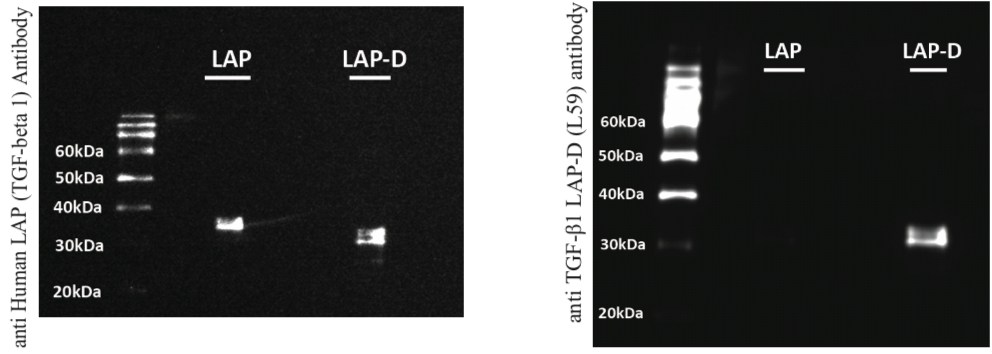 |
| Immunoblot analysis of anti TGF-β1 LAP-D (L59) antibody specificity. Uncut human recombinant LAP (R&D Systems, 246-LP-025; LAP), PLK digested human recombinant LAP; LAP-degradates (LAP-D); 10 ng/lane. (Left) anti Human LAP (TGF-beta 1: R&D Systems, AF-246-NA, 0.1 μg/mL), Peroxidase AffiniPure F(ab')2 Fragment Rabbit Anti-Goat IgG (H+L) (Jackson ImmunoResearch Laboratories, 305-036-045, 0.08 μg/mL) (Right) anti TGF-β1 LAP-D antibody (L59, 10 μg/mL), Peroxidase AffiniPure F(ab')2 Fragment Goat Anti-Mouse IgG (H+L)(Jackson ImmunoResearch Laboratories, 115-036-062, 0.08 μg/mL) Detection: Pierce® ECL Plus Western Blotting Substrate (ThermoFischer Scientific, NCI32132JP) * anti TGF-β1 LAP-D (L59) antibody specificity has been confirmed by detection of LAP-D (31 kDa) |
|
| Figure 3 | 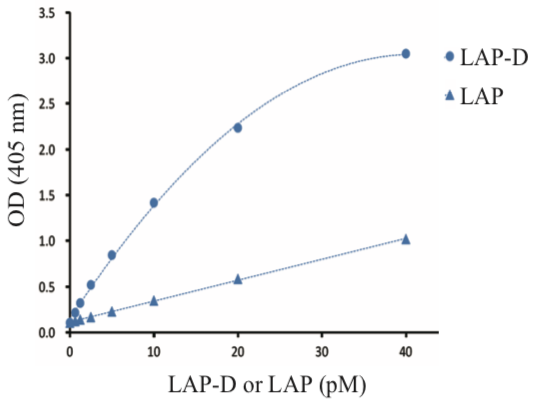 |
| ELISA analysis of specificity of anti TGF-β1 LAP-D (L59) antibody and anti Human LAP TGF-beta 1 biotinylated antibody. Sample: PLK digested human recombinant LAP (L59 LAP degradates, LAP-D), uncut human recombinant LAP (LAP) Coating Antibody: anti TGF-β1 LAP-D (L59) antibody (20 μg/mL) Detection Antibody: anti Human LAP TGF-beta 1 biotinylated antibody (R&D Systems, BAM2462, 0.5 μg/mL) Detection: Streptavidin-AP (Jackson ImmunoResearch Laboratories, 016-050-084, 0.05 μg/mL) * This ELISA system detected uncut human recombinant LAP, which showed c.a. 1 OD at 40 pM, while LAP-D showed c.a. 3 OD at 40 pM, giving significant differences. This result proves the specificity of anti TGF-β1 LAP-D (L59) antibody. |
|
