 CAC Antibody Collection
CAC Antibody Collection
The antibodies on this page are part of Cosmo Bio's exclusive CAC Collection. For many many thousands of other antibodies from many different makers, use our Search the Store function and our Explore Products drop down menu.
Bacteria-related
Antibodies were invented by nature primarily to fight infections caused by microbes. Indeed, before the introduction of childhood vaccinations and antibiotics, serum therapy was used for the treatment of several severe bacterial infections. Passive immunization with hyper-immune serum was introduced already at the end of the 19th century by Emil Behring’s laboratory for the treatment of diphtheria and tetanus. Patients received horse sera generated by immunization with toxoids of Corynebacterium diphteriae and Clostridium tetani, respectively. Since the pathogenesis of diphtheria and tetanus is primarily driven by highly potent toxins, their neutralization by specific serum antibodies resulted in greatly efficacious treatment that was widely used in spite of the risk of serum sickness. Anti-tetanus serum still has an important role in post-exposure prophylaxis, despite the availability of tetanus vaccines and effective antibiotics. Serum therapy was applied to other invasive bacterial infections as well, for example caused by Streptococcus pneumoniae (Pneumococcus) and Staphylococcus aureus. For these two pathogens, serum therapy was less effective than in case of diphtheria and tetanus, likely reflecting a more complex disease pathogenesis, as well as higher heterogeneity of the pathogens. In any case, upon the introduction of effective antibiotics from the 1940s, anti-bacterial serum therapies were largely abandoned. After half a century of highly productive antibiotic development, however, it has now become obvious that antibiotics cannot provide the ultimate solution in the fight against bacterial infections. The non-critical use of antibiotics in human and veterinary medicine has caused widespread resistance in bacteria. Active immunization is certainly a great option to prevent infectious diseases and intensive efforts have been made towards novel vaccines addressing antibiotic resistant bacterial infections, most intensively against S. aureus. Undoubtedly, the vaccine industry faces more difficulties dealing with these antibiotic resistant (nosocomial) microbes – most of them opportunistic pathogens that have intensive interaction and symbiosis with their human host– than strictly pathogenic organisms with well defined, often less complex pathogenesis (e.g. single toxin action). The added difficulty of such vaccine approaches is the target population that is often suboptimal for generating protective immune responses due to chronic underlying diseases, acute conditions, immune compromise and immune senescence. Thus, perhaps unsurprisingly, in looking for answers to address the failing antibiotic pipeline, serum therapy is now being reinvented in modern biotechnology terms, in the form of monoclonal antibodies. In the last two decades, many successful mAb based products were introduced for cancer, autoimmune and inflammatory diseases; however, only one anti-infective mAb has so far made it to market (Synagis, for the prevention of severe Respiratory Syncytial Virus infections of neonates). [from: Oleksiewicz MB., Nagy G., Nagy E., Anti-bacterial monoclonal antibodies: Back to the future? (2012) Archives of Biochemistry and Biophysics. 526(2): 124-131.]
| Product name (click for order info) | Cat No (click for datasheet) |
Host | Species specificity |
| Anti Nephritis-Associated Plasmin receptor (NAPlr/Streptococcal GAPDH) pAb (Rabbit, Purified Ig) | CAC-TMU-PA002 | RAB | Streptococcus |
| Product name | Anti Nephritis-Associated Plasmin receptor (NAPlr/Streptococcal GAPDH) pAb (Rabbit, Purified Ig) |
| Cat No | CAC-TMU-PA001X |
| Description | Acute post-streptococcal glomerulonephritis (APSGN) is an infection- triggered glomerulonephritic disease. Nephritis-Associated Plasmin receptor (NAPlr) is an intracellular component of hemolytic streptococcus that induces nephritis. Glomerular NAPlr deposition is frequently found in infection-related glomerulonephritis (IRGN), especially in glomerulonephritis that is triggered by streptococcal infection (i.e. streptococcal-infection related nephritis [SIRN]). Glomerular NAPlr deposition is not found in non-infection-related nephritis. Therefore, immunostaining using anti-NAPlr antibody is ideal for identifying IRGN and SIRN. Although a distinct commercially available anti-NAPlr monoclonal antibody (1F10) has been in use, it is claimed to suffer from low sensitivity in Immunostaining. By contrast, staining sensitivity is better with polyclonal anti-NAPlr antibodies. However, stable generation and supply of this pAb is difficult because of the complicated step of NAPlr extraction from streptococcal particles. This new polyclonal antibody against an NAPlr peptide was established to balance both satisfactory sensitivity in Immunostaining with stable antibody production. References: 1) Oda T, Up-to-date findings on infection-related glomerulonephritis. 東医大誌73(4):355-363, 2015. 2) Yoshizawa N, Yamakami K, Fujino M, et al. (2004) Nephritis-associated plasmin receptor and acute poststreptococcal glomerulonephritis. J Am Soc Nephrol. 15:1785-93. 3) Oda T, Yamakami K, Yoshizawa N, et al. (2005) Glomerular plasmin-like activity in relation to nephritis-associated plasmin receptor in acute poststreptococcal glomerulonephritis. J Am Soc Nephrol. 16:247-54. 4) Oda T, Yoshizawa N, Yamakami K, et al. (2010) Localization of nephritis-associated plasmin receptor in acute poststreptococcal glomerulonephritis. Hum Pathol. 41(9):1276-85. 5) Oda T, Yoshizawa N, Yamakami K, et al. (2012) The role of nephritis-associated plasmin receptor (NAPlr) in glomerulonephritis associated with streptococcal infection. J Biomed Biotechnol. 2012:417675. |
| Host | RAB |
| Species specificity | Streptococcus |
| Figure 1 | 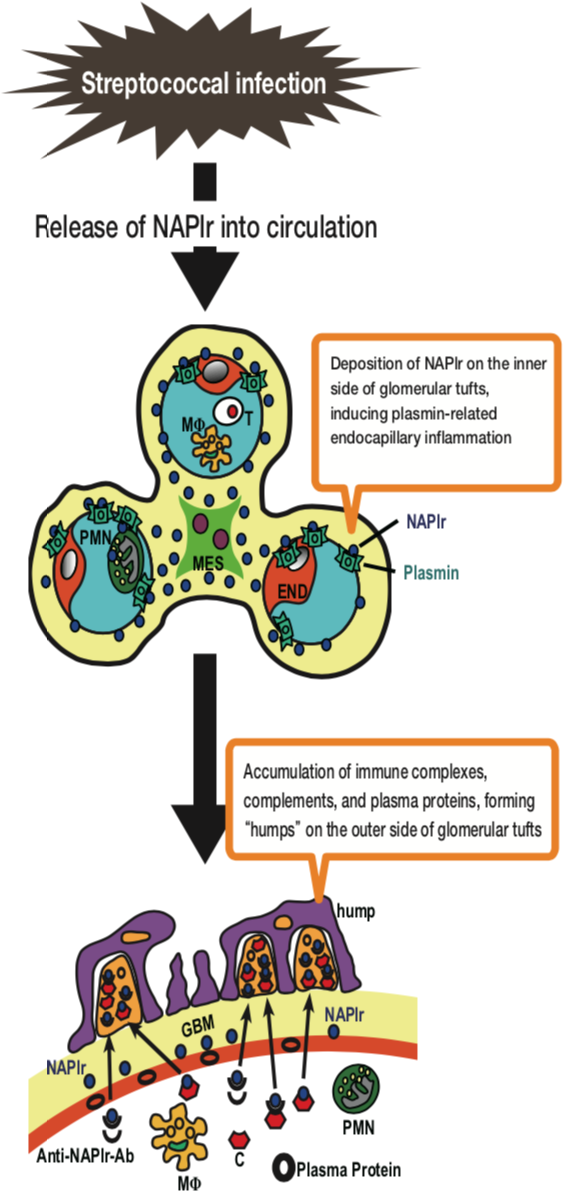 |
| Schematic representation of proposed mechanisms involved in the development of APSGN. MES: mesangial cell; END: endothelial cell; PMN: polymorphonuclear cell; MΦ: macrophage; T: T lymphocyte; GBM: glomerular basement membrane; C: complement; Anti-NAPlr-Ab: Anti-NAPlr-antibody. | |
| Figure 2 | 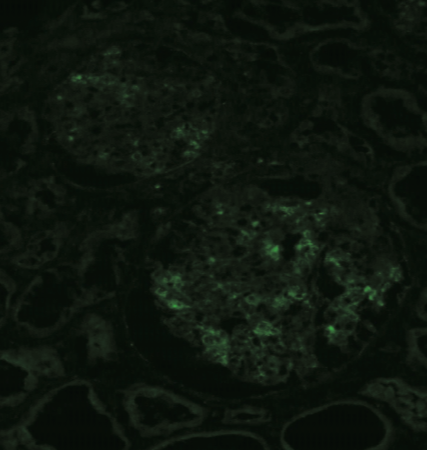 |
| Immunostaining with anti NAPlr-FITC antibody. Primary Antibody: anti NAPlr-FITC, 1:5 dilution [0.26 mg/mL]). Tissue: Section of unfixed frozen renal biopsy tissue from an APSGN patient (positive control). 1. Air dry unfixed renal biopsy frozen section. 2. Wash with PBS (3 min each x 2). 3. 1-hour ambient temperature incubation with primary antibody (anti NAPlr-FITC, 1:5 dilution [0.26 mg/mL]). 4. Wash with PBS (5 min each x 3). 5. Mount with mounting medium for fluorescent staining. 6. Observe stained sections by fluorescent microscope. |
