Huntington’s disease (HD) is a rare, autosomal dominant, progressive neurodegenerative disorder caused by a CAG trinucleotide repeat expansion in the HTT gene on chromosome 4, leading to the production of mutant huntingtin (mHTT) protein with an abnormally long polyglutamine (polyQ) tract. Clinically, HD is characterized by a triad of motor dysfunction, cognitive decline, and psychiatric disturbances, typically manifesting between the ages of 30 and 50 and worsening steadily over 10 to 25 years.
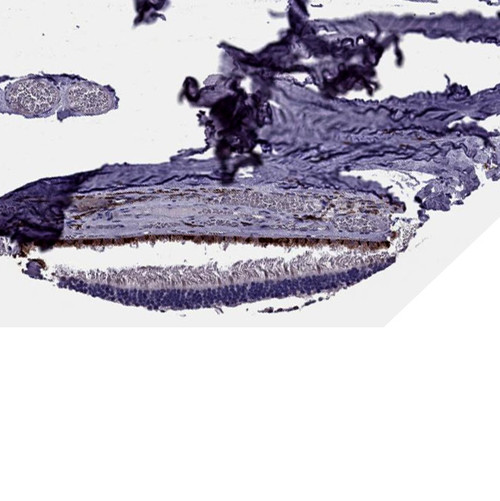
Pathologically, HD is defined by selective neurodegeneration, most prominently in the striatum—especially medium spiny neurons—but also involving the cortex, hippocampus, and other brain regions as the disease advances. At the cellular level, mHTT misfolds and forms aggregates that disrupt multiple physiological pathways including transcriptional regulation, mitochondrial function, axonal transport, autophagy, synaptic transmission, and proteostasis. Dysregulation of various kinases such as CDK5 and MAPKs, mitochondrial energy deficits, excitotoxicity through dysregulated glutamate signaling, and impaired calcium handling all contribute to progressive neuronal dysfunction and death. Moreover, astrocytic and microglial activation contributes to a chronic neuroinflammatory state that exacerbates neurodegeneration.
Unlike other neurodegenerative diseases, HD is monogenic, which has enabled the development of disease models and therapeutic strategies targeting the root genetic cause. Advances in RNA-based therapies, gene editing, and antisense oligonucleotides hold promise for modifying disease progression, though challenges remain in delivery, specificity, and long-term safety. The complexity of HD lies in the diverse cellular roles of huntingtin and the widespread disruption caused by its mutant form, making HD a prototypical model for studying toxic gain-of-function proteinopathies and neurodegeneration.
Browse our complete offering of antibodies, proteins, and assay kits for studying Huntington’s Disease below.










![PPIs Detection Reagent : Fluoppi [ CDK5-p25 ] PPIs Detection Reagent : Fluoppi [ CDK5-p25 ]](https://cdn11.bigcommerce.com/s-ydswqc5qsc/images/stencil/500x659/products/105003/240624/mbl-am-p0014_ppis-detection-reagent--fluoppi--cdk5-p25-_124085__56691.1751948310.jpg?c=2)









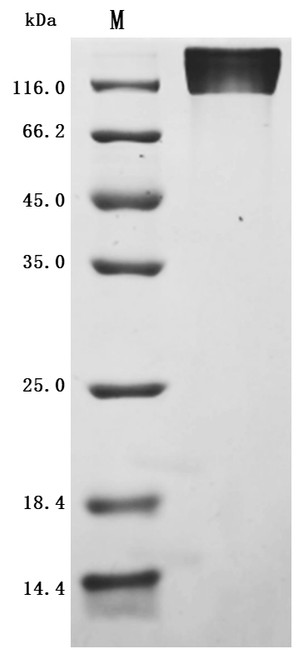
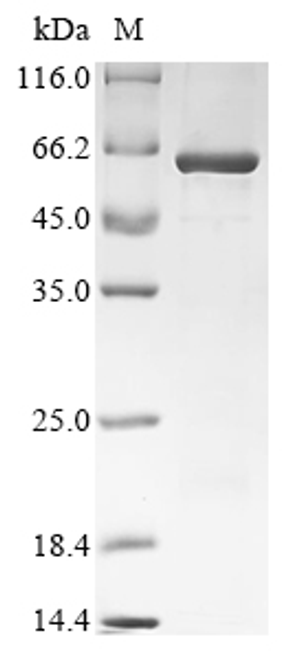


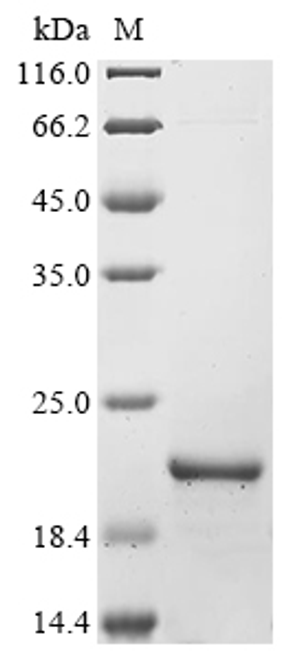


![Lane 1: Marker [kDa] 250, 130, 95, 72, 55, 36, 28, 17, 10 | Lane 2: RT4 | Lane 3: U-251 MG | Lane 4: Human Plasma | Lane 5: Liver | Lane 6: Tonsil Lane 1: Marker [kDa] 250, 130, 95, 72, 55, 36, 28, 17, 10 | Lane 2: RT4 | Lane 3: U-251 MG | Lane 4: Human Plasma | Lane 5: Liver | Lane 6: Tonsil](https://cdn11.bigcommerce.com/s-ydswqc5qsc/images/stencil/500x659/products/101576/181707/atl-hpa076133_anti-chd8-pab-atl-hpa076133_63375__12440.1681138094.jpg?c=2)