Navigation
Neurodegeneration Products Dashboard
Aβ
tau
α-synuclein (this page)
TDP-43
C9ORF72
Huntingtin
Ordering Information
Neurodegeneration-related Flyers and Brochures
Antibodies for Neurodegeneration Research (TDP-43, 4R-tau, C9ORF, Synuclein)
PDF Download
Oxidative Stress Markers
mAbs and Selection Guide
PDF Download
Autophagy/Mitophagy Antibodies
PDF Download
Glutamate and NMDA related
Dopamine related
Cholesterol
Ganglioside
Glucosylceramide
Sphingosine
Mitochondria related
Heat shock
Oxidative stress
DNA damage
Lipid peroxidation
Protein carbonylation
Senescence
Transcription and Chromatin
Autophagy
Nucleocytoplasmic transport
Prion protein
APOETREM2
Progranulin
SOD1
FUS
Humanin
Gfap
Paraoxonase
CD36
Dysbindin
BDNF
VEGF
PARP
PPAR gamma
Ubiquitin
Proteasome
Proteases
Caspases
Peptidyl-prolyl cis-trans isomerases
NADPH oxidases
Dyneins
Kinesins
Kinases and Kinase inhibitors
Signaling molecules
Nuclear Hormone Receptors
Products for Neurodegeneration Research: α-synuclein
α-synuclein Antibodies and Kits (click catalog number for product and ordering information)
| Catalog Number | Product Name | Specificity | Size |
| CAC-TIP-SN-P01 | Anti Alpha Synuclein (Amino Acids 1-10) pAb (Rabbit, Antiserum) | Human, Mouse | 50UL |
| CAC-TIP-SN-P02 | Anti Alpha Synuclein (Amino Acids 11-20) pAb (Rabbit, Antiserum) | Human, Mouse | 50UL |
| CAC-TIP-SN-P03 | Anti Alpha Synuclein (Amino Acids 21-30) pAb (Rabbit, Antiserum) | Human, Mouse | 50UL |
| CAC-TIP-SN-P04 | Anti Alpha Synuclein (Amino Acids 31-40) pAb (Rabbit, Antiserum) | Human, Mouse | 50UL |
| CAC-TIP-SN-P05 | Anti Alpha Synuclein (Amino Acids 41-50) pAb (Rabbit, Antiserum) | Human, Mouse | 50UL |
| CAC-TIP-SN-P06 | Anti Alpha Synuclein (Amino Acids 51-60) pAb (Rabbit, Antiserum) | Human, Mouse | 50UL |
| CAC-TIP-SN-P07 | Anti Alpha Synuclein (Amino Acids 61-70) pAb (Rabbit, Antiserum) | Human, Mouse | 50UL |
| CAC-TIP-SN-P08 | Anti Alpha Synuclein (Amino Acids 75-91) pAb (Rabbit, Antiserum) | Human, Mouse | 50UL |
| CAC-TIP-SN-P09 | Anti Alpha Synuclein (Amino Acids 131-140) pAb (Rabbit, Antiserum) | Human, Mouse | 50UL |
| CAC-TIP-SN-SET | Anti Alpha Synuclein (9 Antibody Set) pAb (Rabbit, Antiserum) | Human, Mouse | 9x10UL |
| CSB-E18033h | Human Alpha Synuclein (non A4 component of amyloid precursor) oligomer (SNCA oligomer) ELISA kit | Human | 1x96 rxns |
| CSB-EL021912RA | Rat Alpha-synuclein (SNCA) ELISA kit | Rat | 1x96 rxns |
| CSR-SYN01 | Alpha Synuclein Aggregation Assay Kit | - | 1 Kit |
| CSR-SYN03 | Alpha Synuclein Fibrils | Human | 0.1MG |
| CSR-SYN04-2 | Alpha Synuclein | Human | 0.1MG and 1MG |
| CSB-EL010868HU | Human heparan sulfate proteoglycan 2 (HSPG2) ELISA kit | Human | 1x96 rxns |
| CSB-E14983h | Human Syndecan-1/CD138(SDC1) ELISA Kit | Human | 1x96 rxns |
| CSB-EL020889HU | Human Syndecan-2(SDC2) ELISA kit | Human | 1x96 rxns |
| CSB-EL020888MO | Mouse Syndecan-1(SDC1) ELISA kit | Mouse | 1x96 rxns |
| CSB-EL020889MO | Mouse Syndecan-2(SDC2) ELISA kit | Mouse | 1x96 rxns |
| CSB-E17413p | Pig Syndecan-1/CD138(SDC1) ELISA Kit | Pig | 1x96 rxns |
| CSB-EL010868RA | Rat heparan sulfate proteoglycan 2 (HSPG2) ELISA kit | Rat | 1x96 rxns |
| CSB-E17115r | Rat Syndecan-1/CD138(SDC1) ELISA Kit | Rat | 1x96 rxns |
| CSB-EL020889RA | Rat Syndecan-2(SDC2) ELISA kit | Rat | 1x96 rxns |
| CAC-NU-07-004 | Anti Syndecan-3 (N-Syndecan/Neuroglycan) pAb (Rabbit, Antiserum) | Mouse, Rat | 100UL |
| CSB-E11834h | human S100 calcium binding protein A9/calgranulin B,S100A9 ELISA Kit | Human | 1x96 rxns |
| CSB-EL020642MO | Mouse Protein S100-A9(S100A9) ELISA kit | Mouse | 1x96 rxns |
| CSB-EL020642RA | Rat Protein S100-A9(S100A9) ELISA kit | Rat | 1x96 rxns |
| YMS-80126-EX | S100A9 Assay Kit | Rat | 96 tests |
| YMS-80086 | Anti S100A9 mAb (Clone 15E9) | Rat | 200UG |
Just as pathological tau protein inclusions define the Tauopathies, the synucleinopathies are neurodegenerative diseases defined by deposition of α-synuclein aggregates in neuronal and non-neuronal cells in the brain. The classic synucleinopathies are PD, dementia with Lewy bodies (DLB) and multiple systems atrophy (MSA). The defining histopathological features in PD are spindle- or thread-like Lewy neurites (LNs) in neuronal processes and globular Lewy bodies (LBs) in neuronal perikarya. Just as some APP mutations cause FAD, some mutations in SNCA (for example, E46K and A53T) are associated with familial PD. Please scroll to the ORDERING section to explore CosmoBio USA's α-synuclein reagents that have proven helpful in the study of neurodegenerative diseases.
α-Synuclein is a small 140 amino acid protein encoded by the SNCA gene on chromosome 4q22.1. It is found in many neuronal compartments and is particularly abundant in presynaptic terminals, where it may be involved in vesicle trafficking and neurotransmitter release. It is intrinsically flexible in solution and, like other intrinsically disordered proteins (IDPs), acquires structure upon binding to biological partners, including lipid membranes which appear to play a key role in α-synuclein function and dysfunction. While α-synuclein has been associated with several biological activities, including regulation of synaptic vesicle pools, neurotransmitter release, SNARE complex assembly and vesicle trafficking, its precise functions remain enigmatic and controversial.
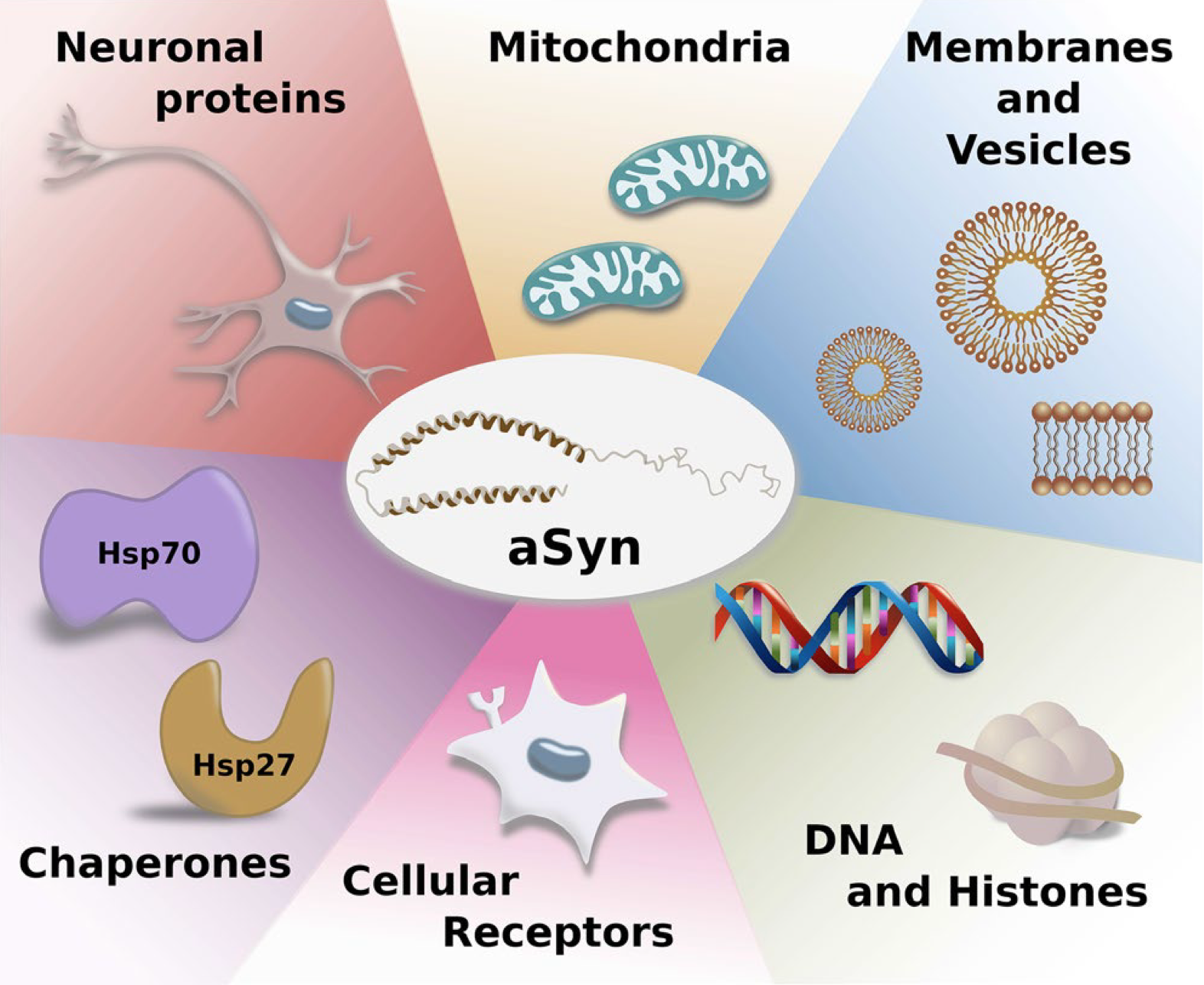
 From: Brás, I., Dominguez‐Meijide, A., Gerhardt, E., Koss, D., Lázaro, D., Santos, P., Vasili, E., Xylaki, M., Outeiro, T. (2020). Synucleinopathies: Where we are and where we need to go Journal of Neurochemistry 153(4), 433-454.
From: Brás, I., Dominguez‐Meijide, A., Gerhardt, E., Koss, D., Lázaro, D., Santos, P., Vasili, E., Xylaki, M., Outeiro, T. (2020). Synucleinopathies: Where we are and where we need to go Journal of Neurochemistry 153(4), 433-454.
Many factors contributing to α-synuclein aggregation are linked to its structure. α-Synuclein is composed of three distinct domains: N-terminal region contains four imperfect KTKEGV motif repeats that can fold into amphipathic helices. The central non-amyloid component domain is hydrophobic and is crucial for α-synuclein aggregation and C-terminal region is enriched in highly acidic and charged amino acids. α-Synuclein is classified as an intrinsically disordered protein that may exist as an un-structured monomer. Upon membrane/lipid binding, the N-terminal region adopts an alpha-helical structure that may promote membrane curvature. α-Synuclein mutations associated with familial PD (A30P, E46K, H50Q, G51D or A53T) are found mostly at the N-terminal helix and lead to accelerated fibrillization and increased toxicity. These mutation-mediated effects resulted from altered α-synuclein secondary structure (A30P, A53T); enhanced α-synuclein binding to phospholipids (E46K); and from formation of α-synuclein fibrils more prone to activate proapoptotic pathways (G51D).
The non-amyloid component domain of α-synuclein comprises the 35 residue (aa61-95) central hydrophobic region and plays a major role in α-synuclein oligomerization and aggregation. This hydrophobic motif may become exposed during misfolding and initiate aggregation. Mutation or deletion of this region significantly reduced α-synuclein filament assembly.
Proteolytic cleavage can alter α-synuclein structure to favor aggregation. Thus, C-terminal truncation of α-synuclein is a common post-translational modification abundant in Lewy bodies. Tissue-specific expression of C-terminally truncated α-synuclein in nigral dopaminergic neurons markedly reduced dopamine levels in a transgenic mouse model, suggesting a failure to maintain α-synuclein-regulated dopamine homeostasis.
Hyperphosphorylation of α-Synuclein impaired multiple aspects of its function. α-Synuclein phosphorylation at S129 and Y125 resulted in reduced interactions with proteins involved in the mitochondrial electron transport chain complex. At the same time these PTMs enhanced interactions with proteins involved in cytoskeletal organization, vesicular trafficking and serine phosphorylation. Not all phosphorylation promoted aggregation. For example, S87 phosphorylation, which increased the conformational flexibility of α-synuclein and lead to reduced binding affinity to lipid membranes, also reduced the potential of α-synuclein to form fibrils. These studies have been assisted by antibodies against specific phospho-epitopes that could recognize hyperphosphorylated α-synuclein in LBs.
Other pathways to accumulation of α-synuclein aggregates were indirectly promoted by oxidative stress, fatty acids (i.e., glucosylceramide) and impaired protein clearance mechanisms. Proteins with chaperone activity towards α-synuclein, such as Hsp70 and several other heat-shock proteins, and some presynaptic proteins like Munc18-1 regulated α-synuclein aggregation and toxicity. In addition, α-synuclein-interacting enzymes, such as peptidyl prolyl isomerases FKBP12, FKBP52 and PREP (prolyl oligopeptidase) promoted α-synuclein oligomerization and aggregation.
The formation and spread of α-synuclein pathology have been extensively studied using the intracerebral seeding method. Human α-synuclein, extracted from post mortem PD patient brains, was capable of seeding intracellular accumulation of phosphorylated α-synuclein in both monkeys and in WT mice, though without the formation of clear LB-like inclusions or with LB-like inclusions only present in certain brain regions such as the amygdala in about half of injected mice. Human brain-derived α-synuclein from patients with MSA or incidental LBD (Lewy body pathology without clinical symptoms of dementia or Parkinsonism) was also effective at seeding hyperphosphorylated α-synuclein pathology in transgenic mice that modestly overexpressed WT human α-synuclein in the absence of murine α-synuclein. Seeding with extracts from pathology laden brains of aged α-synuclein transgenic mice (line M83) accelerated the onset of α-synuclein pathology in young pre-symptomatic M83 hosts, much like what has been observed following seeding of appropriate hosts with Aβ or tau derived from the brains of transgenic mice. Seeded α-synuclein pathology took the form of LB- and LN-like intracellular deposits, resembling the pathology that emerges spontaneously in PD and DLB. Further, the deposits were recognized by conformation-dependent antibodies against α-synuclein and ubiquitin and were stained by the amyloidophilic dye Thioflavin S (ThS). These results resemble how, in WT hosts, tau derived from patients with AD or other Tauopathies could seed intracellular accumulation of pathological, hyperphosphorylated tau that eventually progressed to formation of insoluble aggregates, strengthening the theory that prion-like templated misfolding contributes to neurodegenerative disease-associated protein deposition.
(Adapted from: Birol, M., Melo, A. (2020). Untangling the Conformational Polymorphism of Disordered Proteins Associated With Neurodegeneration at the Single-Molecule Level Frontiers in Molecular Neuroscience 12(), 309; Valera, E., Spencer, B., Masliah, E. (2016). Immunotherapeutic Approaches Targeting Amyloid-β, α-Synuclein, and tau for the Treatment of Neurodegenerative Disorders Neurotherapeutics 13(1), 179-189; Yan, X., Uronen, R., Huttunen, H. (2018). The interaction of α-synuclein and tau: A molecular conspiracy in neurodegeneration? Seminars in Cell & Developmental Biology 99(), 55-64; McAllister, B., Lacoursiere, S., Sutherland, R., Mohajerani, M. (2020). Intracerebral seeding of amyloid-β and tau pathology in mice: factors underlying prion-like spreading and comparisons with α-synuclein Neuroscience & Biobehavioral Reviews 112(), 1-27; and Brás, I., Dominguez‐Meijide, A., Gerhardt, E., Koss, D., Lázaro, D., Santos, P., Vasili, E., Xylaki, M., Outeiro, T. (2020). Synucleinopathies: Where we are and where we need to go Journal of Neurochemistry 153(4), 433-454)

