ACE test (Dry Heat Kit)
Fukuzawa Shoji
- Catalog No.:
- FUK-H6302K
- Shipping:
- Calculated at Checkout
$465.00
Product Description
Biological indicator for sterilization validation
For Validation and Monitoring of Sterilization Processes. ACE test is a biological indicator which can confirm whether sterilization has been carried out properly after autoclave, EO Gas, H2O2, LTSF, formalin, and steam sterilization at 115°C
Features
- Highly reliable sterilization results obtained after 24 hours, 48 hours or 7 days culturing
- Built-in chemical indicator provides quick identification of processed BIs
- Potency and stability retained for 18 months after production
- Fits competitorʼs incubators
- Matches criteria required as a biological indicator for JP, USP, EN866, and ISO11138 Sterilization methods
| Product | Spore | Usage |
| Biological Indiator for Steam FUK-H3723T |
Geobacillus stearothermophilus | A self-contained biological indicator for monitoring steam sterilization of 121℃, 132℃ |
| Biological Indiator for 115℃ Steam FUK-H6301 |
Bacillus subtilis "5230" | A self-contained biological indicator for monitoring steam sterilization of 115℃ |
| Biological Indicator for Ethylene Oxide FUK-H3724 |
Bacillus atrophaeus | A self-contained biological indicator for monitoring ethylene oxide sterilization |
| Biological Indicator for Hydrogen Peroxide Gas/Plasma FUK-H3725T-25 |
Geobacillus stearothermophilus | A self-contained biological indicator for monitoring hydrogen peroxide (Gas/Plasma) sterilization |
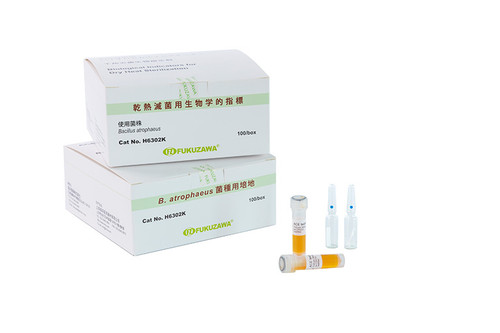
| Biological Indicator for Dry Heat FUK-H6302K |
Bacillus atrophaeus | A glass ampoule biological indicator for monitoring dry heat sterilization of 160~350℃ (with culture medium) |
Why ACE test®?
With the ACE test® you can:
• Validate sterilization processes reliably across different modalities with one unified BI line.
• Simplify monitoring with integrated chemical indicators plus spore media — reducing steps and potential error.
• Maintain compliance with recognized international sterilization‐validation standards.
• Provide end-users, QC labs and regulatory auditors with documented proof of sterilization efficacy.
| Documents & Links for ACE test (Dry Heat Kit) | |
| Flyer | ACE test (Dry Heat Kit) Flyer |
| Documents & Links for ACE test (Dry Heat Kit) | |
| Flyer | ACE test (Dry Heat Kit) Flyer |