Other related information : Hemagglutination activity assay
Importance of glycans and significance of glycan research
Since 1980, basic research on sugar chains has progressed rapidly, and sugar chains play important roles in and outside the body.
A number of examples have been reported (table below). While these basic researches will continue to be actively pursued in the
future, applied research using them is also progressing gradually. One example is the application to diagnosis and treatment.
Methods for preventing and treating infections by controlling the function of sugar chains (glycopharmaceuticals, artificial proteins)
and methods for diagnosing disease-specific changes in sugar chains are being developed
Hemagglutination is the most common method of measuring lectin activity. In this way, lectins from various sources can be easily screened. Furthermore, when aggregation is inhibited by the addition of sugar or glycoprotein, the sugar-binding specificity of the lectin can be determined, which is an essential technique in lectin research. Lectin activity is expressed as the maximum dilution factor that causes hemagglutination. (It can be said that the higher the dilution ratio, the stronger the lectin activity.) In addition, when the concentration of the lectin solution is known, the strength of activity can be estimated at the lowest concentration of lectin that causes hemagglutination (χμg/mL) . It can also be displayed numerically. (It can be said that the smaller the minimum concentration value, the stronger the lectin activity.)
Human or rabbit red blood cells are usually used, but sometimes red blood cells pretreated with trypsin or sialidase are used. These treatments may expose or eliminate sugar chains recognized by lectins, altering agglutinating activity and thus providing additional information.
Measuring method
1. Transfer 2-3 mL of stored blood into a screw cap test tube, add 4-5 times the volume of PBS, centrifuge at 2,000 rpm for 5 minutes, and discard the supernatant. This operation is repeated 3 to 4 times to obtain a red blood cell fraction from the stored blood.
2. Add PBS to 1. to make a 2% (v/v) erythrocyte suspension. (If red blood cells are 0.2mL, dilute to 10mL with PBS.)
3. Dispense 25 μL of PBS into rows 2-11 of each well of a 96-well titer plate (U-bottom) .
4. Pour 50 μL of the lectin solution into the first row of a 96-well titer plate, take 25 μL of the lectin solution from the first row, transfer it to the adjacent well on the right (second row), and stir by pipetting. Repeat this operation up to the 11th row to create a 2-fold dilution series.
5. Divide 50μL of 2% (v/v) erythrocyte suspension into columns 1 to 11 and leave at room temperature for 30 to 60 minutes.
6. Determine the presence or absence of agglutination based on the state of sedimentation of erythrocytes to the bottom of each well.

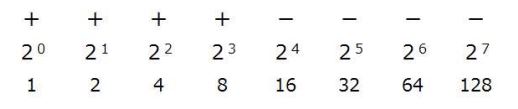
The activity indicates the lowest concentration of lectin capable of agglutinating erythrocytes as the final concentration on the titer plate. In the figure above, the maximum dilution of lectin that can agglutinate erythrocytes is 23 (=8). When the initial lectin concentration is 0.5 mg/mL, the lowest lectin concentration that causes hemagglutination is calculated as follows.
0.5 (mg/mL) ÷ 3 ÷ 23 = 0.0208 mg/mL (= 20.8 μg /mL)
Method for preparing trypsinized red blood cells
Add 5 volumes of PBS, centrifuge at 2,000 rpm for 5 minutes, and discard the supernatant. This operation is repeated 3-4 times to obtain a red blood cell fraction (about 200-300 μL ) from the stored blood.
Method for preparing trypsinized erythrocytes
1. Take 2-3 mL of stored blood in a screw cap test tube, add 4-5 times the volume of PBS, and rotate at 2,000 rpm for 5 minutes.
After centrifugation, discard the supernatant.
2. add 400 μL of PBS with a micropipette , and dissolve. (It becomes slightly cloudy, but confirm that there is no precipitate.) (enzyme concentration 0.5%)
3. Add an equal volume of trypsin solution to the erythrocyte fraction (approximately 200-300 μL ), stir gently, and allow to stand in a 37° C. water bath for 10 minutes.
4. Add PBS, centrifuge at 2,000 rpm for 5 minutes, and discard the supernatant. Add PBS again and stir. Repeat this operation 3-4 times.
Add PBS to the obtained erythrocyte fraction (200 μL ) to make a 2% (v/v) trypsinized erythrocyte solution. With agglutination: erythrocytes do not sediment and spread throughout the well (+). No agglutination: Sedimented erythrocytes are small dots (-). Dilution ratio on well = lectin solution 25 µL + red blood cell solution 50 µL 3/3
Method for preparing sialidase-treated erythrocytes
1. Transfer 2-3 mL of stored blood into a screw cap test tube, add 4-5 times the volume of PBS, centrifuge at 2,000 rpm for 5 minutes, and discard the supernatant. This operation is repeated 3-4 times to obtain a red blood cell fraction (about 200-300 μL ) from the stored blood.
2. Sialidase (Neuraminidase) ( Arthrobactor ureafaciens ) is dissolved by adding 1 mL of pure water with a micropipette. (Enzyme concentration 1 unit/mL) Further dilute the sialidase solution 10-fold with PBS. (Enzyme concentration 0.1 unit/mL)
3. Add about 2 mL of PBS to the red blood cell fraction (about 200-300 μL ) and stir with a Pasteur pipette. Further, add 400 μL of the prepared sialidase solution with a micropipette and gently stir with a Pasteur pipette. Allow to stand in a 37°C water bath for 60 minutes.
4. Add PBS, centrifuge at 2,000 rpm for 5 minutes, and discard the supernatant. Add PBS again and stir. Repeat this operation 3-4 times.
Add PBS to the obtained erythrocyte fraction (200 µL ) to make a 2% (v/v) sialidase-treated erythrocyte solution.
References
1) Karlsson, K.-A., Annu. Rev. Biochem., 58, 309 (1989)
2) Mouricout, M., et al., Infect. Immun., 58, 98 (1990)
3) Gottschalk, A., Drzemitz, R., Glycoproteins, 2nd ed., 381, Elsevier (1972)
4) Suzuki, Y., et al., Virology, 189, 121 (1992)
5) Leonard, CK, et al., J. Biol. Chem., 265, 10373 (1990)
6) Mizuochi, T., et al., Biochem. J., 254, 599 (1988)
7) Chanh, TC, et al., Proc. Natl. Acad. Sci., 84, 3891 (1987)
8) Parkins, ME, Rocco, LJ, J. Immunol., 141, 3190 (1988) Literature
9) Cuatrecasas, P., Biochemistry, 12, 3547 (1973)
10) Motoki Hoshi, Spermology, 168, University of Tokyo Press (1992)
11) Muramatsu, T., J. Cell. Biochem., 36, 1 (1988)
12) Ashwell, G., Hartford, J., Annu. Rev. Biochem., 51, 531 (1982)
13) Kornfeld, S., J. Clin. Invest., 77, 1 (1986)
14) Takada, A., Kannagi, R., et al., Biochem. Biophys. Res. Commun., 179, 713 (1991)
15) Lasky, LA, Rosen, SD, et al., Cell, 69, 927 (1992) 6) Mizuochi, T., et al., Biochem. J., 254, 599 (1988)
