this page: Exosome Dashboard
Product Lineup
Tetraspanin mAbs for exosome purification and characterization
Tetraspanin antibody-based exosome purification and ELISA kit
ExoTrap™(to capture exosomes)
Ultracentrifugation media for exosome purification
OptiPrep™ (for exosome isolation)
Milk exosome reagents
Antibodies and ELISAs for putative exosome markers and negative controls
Other Resources
User Reports
Annotated Bibliography
Exosome Dashboard
User Reports
Nao Nishida-Aoki
National Cancer Center Research Institute of Molecular and Cellular Therapy

Experimental findings
Exosomes are lipid bilayer-delimited vesicles of about 100 nm in diameter. Cancer cells adversely affect surrounding cells by secreting exosomes that promote cancer invasion and metastasis. We wondered whether inhibiting the function of exosomes derived from cancer cells would suppress cancer metastasis. Toward this end, we used antibodies that bind to exosomes to inhibit the action of exosomes derived from cancer cells.
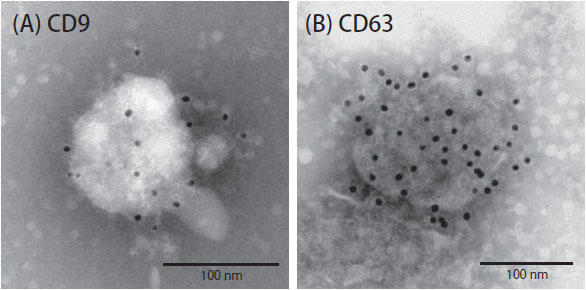
Antibodies that recognize exosomes from human breast cancer cells were sought for use in a mouse xenotransplantation model with human breast cancer cells. First, we wanted to check whether anti-human CD9 and anti-human CD63 would bind to the surface of exosomes isolated from breast cancer cell conditioned media. The small size of exosomes prevented the use of conventional microscopy. Thus, it was necessary to employ immunoelectron microscopy. However, immunoelectron microscopy is challenging and requires antibodies that bind well to their targets.
Anti-human CD9 (clone 12A12) and anti-human CD63 (clone 8A12) have proven track records in immunoprecipitation and were thus promising candidates to test in immunoelectron microscopy. These reagents easily detected CD63. The detection of CD9, however, required substantial protocol modifications before we achieved successful staining (Figure 1). In addition, for the purposes of this experiment, it was important that the chosen antibodies have exquisite specificity for human and not mouse to prevent toxicity and off-target effects. The antibodies were found to be very human-specific in western blot experiments, readily detecting CD9 and CD63 derived from human, but not mice (Figure 2).
Having established detection protocols and the human specificity of both antibodies, it was possible to advance to in vivo studies. Anti-human CD9 (clone 12A12) and anti-human CD63 (clone 8A12) were administered in the murine xenograft model of breast cancer to determine whether it was possible to suppress metastasis. We were able to successfully inhibit cancer metastasis in this model (Reference 1). We consider the success of this experiment to be solely due to the high quality of the antibodies we used. I hope that these antibodies are able to help more people in their studies.
Figures
 |
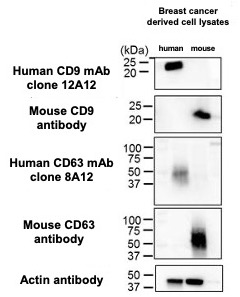 |
| Figure 1. Immunoelectron microscopic detection of human CD9 and CD63 protein on exosome surfaces (A) Anti-human CD9 antibody (clone 12A12) detected CD9 on the surface of exosomes from human breast cancer cells. (B) Anti-human CD63 antibody (clone 8A12) also detected CD63 on the surface of exosomes from human breast cancer cells. | Figure 2. Western blot for human and mouse CD9 and CD63 protein Anti-human CD9 antibody (clone 12A12), anti-human CD63 antibody (clone 8A12), anti-mouse CD9 antibody and anti-mouse CD63 antibody were used to probe blots containing lysates from human breast cancer cell lines (MDA-MB-231-lucD3H2LN) and murine breast cancer cell line (4T1-luc). |
References
- Nishida-Aoki, N., N. Tominaga, F. Takeshita, H. Sonoda, Y. Yoshioka, and T. Ochiya. (2017) Disruption of Circulating Extracellular Vesicles as a Novel Therapeutic Strategy against Cancer Metastasis, Mol Ther, 25: 181-91.
Nobuyoshi Kosaka
National Cancer Center Research Institute of Molecular and Cellular Therapy

Experimental findings
Exosomes are small extracellularly secreted lipid bilayer-delimited vesicles of approximately 100 nm in diameter. The ISEV (International Society for Extracellular Vesicles) recommends the use of the term extracellular vesicles (EVs) to refer to the heterogenous class of secretory vesicles that includes exosomes derived from multivesicular endosomes. Recent exosomes studies have begun to clarify the role of exosomes in some diseases. However, to understand the production mechanism of exosomes it is necessary to have: 1) physiological understanding of the role of exosomes; 2) understanding of the diseases that arise from exosomes. Elucidating the mechanism of exosome secretion by cancer cells may lead to new treatments for cancer.
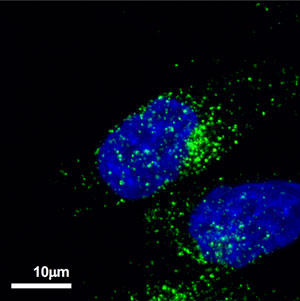
To track the kinetics of exosome production we performed immunostaining experiments with an anti-human CD63 antibody (clone 8A12; available from Cosmo Bio USA). Using immunofluorescence microscopy, we observed a number of CD63 positive granule-like structures in prostate cancer cell line PC-3M (Figure 1) and in colon cancer cell line HCT116 (Figure 2). In the future we will co-stain cancer cells with anti-human CD63 antibody (clone 8A12) and antibodies for other endosomal markers to explore the production of exosomes by cancer cells. The high specificity of anti-human CD63 antibody (clone 8A12) allowed it to be used successfully to target exosomes in vivo to suppress cancer metastasis.
Figures
 |
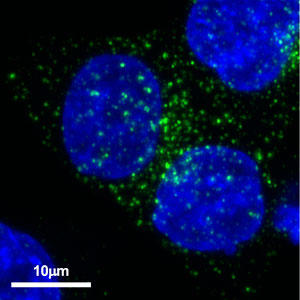 |
| Figure 1. Localization of CD63 in prostate cancer cell line PC-3M Anti-human CD63 antibody (clone 8A12) was detected by Alexa Fluor™ 488-labeled anti-mouse secondary antibody. | Figure 2. Localization of CD63 in colon cancer cell line HCT116 Anti-human CD63 antibody (clone 8A12) was detected by Alexa Fluor™ 488-labeled anti-mouse secondary antibody |
Yusuke Yoshioka
National Cancer Center Research Institute of Molecular and Cellular Therapy

Experimental findings
In 1975 George Kohler and César Milstein invented monoclonal antibody production technology for which in 1984 they were awarded the Nobel Prize in Physiology or Medicine. Monoclonal antibodies have revolutionized the pharmaceutical industry, opening the door to new types of diagnostics and targeted therapies. It has benefited not only medical practice but also basic research. Not all monoclonal antibodies are equivalently useful; it is important to identify "good" antibodies for each specific application. In general, a “good” antibody is one that recognizes a single molecule and binds strongly to its molecular target.
Obtaining a "good antibody" is especially important in exosome research. Exosomes are lipid bilayer-delimited vesicles of approximately 100 nm in diameter that contain various proteins in their lumen as well as in their membranes. Antibodies have been used to detect and isolate exosomes occurring in body fluids or culture supernatants using immunoelectron microscopy, immunoblotting, ELISA, immunoprecipitation and immunoaffinity columns. These applications require “good” antibodies.
As exosomes are heterogeneous, it is likely that, depending on their source, they will display and contain distinct molecules. However, a subset of proteins is likely to be shared across different types of exosomes. Currently, the best pan-exosome markers are the tetraspanins CD9, CD63 and CD81. My exosome research began with the goal of building an exosome detection system. I struggled to find monoclonal antibodies that recognized exosomal markers in my detection system. Although I tried a number of clones, I did not obtain good results until I discovered anti CD9 (clone 12A12) and anti-CD63 (clone 8A12) (Figure 1 and Reference 2). These antibodies performed well in western blots (Figure 2) and immunoelectron microscopy. The human specificity of these antibodies was confirmed by their inability to detect mouse CD9 and mouse CD63. This property is useful in human xenotransplantation model systems in mice where the antibodies do not react with endogenous CD9 and CD63. If problems are encountered in detecting human exosomes, anti-CD9 (clone 12A12) and anti-CD63 (clone 8A12) antibodies may be useful.
Figures
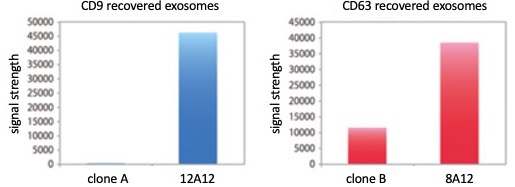 Figure 1. ELISA performance of clones 12A12 (anti-CD9) and 8A12 (anti-CD63) relative to competitor clones A and B.
Figure 1. ELISA performance of clones 12A12 (anti-CD9) and 8A12 (anti-CD63) relative to competitor clones A and B. 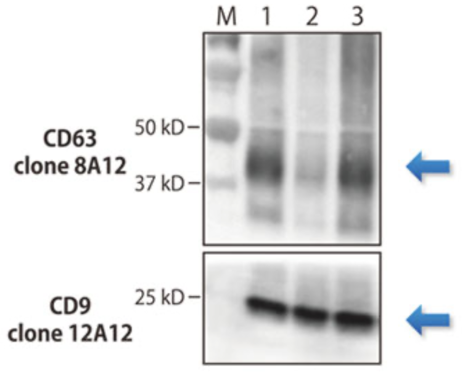 Figure 2. Western blot performance of clones 8A12 (anti-CD63) and 12A12 (anti-CD9) on human exosomes.
Figure 2. Western blot performance of clones 8A12 (anti-CD63) and 12A12 (anti-CD9) on human exosomes. References
- Yoshioka Y et al. (2013) J Extracell Vesicles, 2:20424
- Yoshioka Y et al. (2014) Nat Commun, 5:3591
Akiko Takahashi
Foundation for Research on Cancer

Experimental findings
Exosomes arise by the action of the ESCRT complex from vesicles contained in multivesicular endosomes. These intraluminal vesicles are secreted as extracellular vesicles with diameter of 50-150 nm. Recently, with the discovery that exosomes contain proteins, lipids and nucleic acids, much attention has focused on their function as intercellular communication vehicles.
We are in the process of analyzing the senescence-associated secretory phenotype, characterized in part by elevated secretion of inflammatory cytokines and chemokines. Senescent cells also elevate their secretion of exosomes (Reference 1). We induced cellular senescence by serial passage of normal human fibroblasts (replicative senescence) and by introduction of the activated oncogene RasV12 (oncogene-induced senescence). Following 48 hours of incubation, exosomes in the conditioned medium were recovered by ultracentrifugation. The size distribution of the recovered extracellular material was characterized by Nanoparticle Tracking Analysis. The number of secreted exosomes was 30 to 50 times higher in senescent cells than young cells (Figure 1, top). To further compare senescent versus young exosomes we performed western blotting experiments using an anti-human CD81 monoclonal antibody (clone 12C4, used at 1:1000 dilution; from Cosmo Bio USA). The results showed an increase in the amount of CD81 protein recovered from senescent versus young exosomes (Figure 1, bottom). Exosomes recovered from senescent cells have also been observed to exhibit a growth-promoting effect on MCF-7 breast cancer cells (Reference 2).
The physiological role and diagnostic/therapeutic potential of exosomes are still incompletely understood. We are analyzing the detailed molecular mechanism by which normal and aged cells secrete exosomes, with the hope of clarifying the biological significance of exosomes. Furthermore, by studying the senescence-associated secretory phenotype, I hope to elucidate the roles that cancer exosomes play in age-related diseases.
Figures
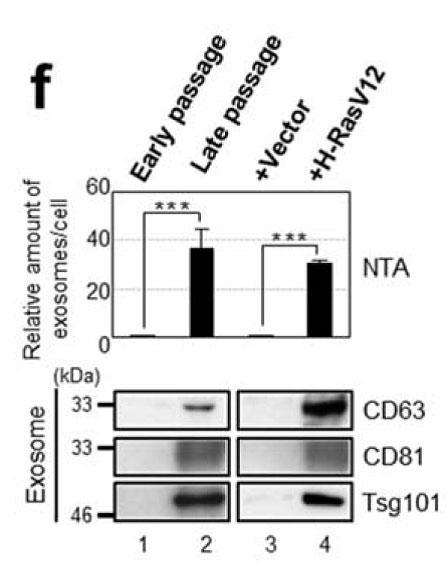 Analysis of exosomes from young and senescent versions of human fibroblast cell line TIG-3 (from Reference 1). Top: Relative number of exosomes recovered as determined by NTA (Nanoparticle Tracking Analysis). Bottom: Western blot detection of exosomal marker proteins.
Analysis of exosomes from young and senescent versions of human fibroblast cell line TIG-3 (from Reference 1). Top: Relative number of exosomes recovered as determined by NTA (Nanoparticle Tracking Analysis). Bottom: Western blot detection of exosomal marker proteins. References
- Takahashi, A. et al. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat Commun 8, 15287 (2017).
- Takasugi, M. et al. Small extracellular vesicles secreted from senescent cells promote cancer cell proliferation through EphA2. Nat Commun 8, 15729 (2017)
Yoshitaka Sato
Department of Virology
Nagoya University Graduate School of Medicine

Experimental findings
Exosomes are membrane-enclosed vesicles of 30 to 100 nm containing proteins and nucleic acids and are secreted by various cells. By gaining access to the circulation, they may transmit signals to cells at distant locations. It has been observed that intercellular communication via exosomes is associated with antigen presentation, apoptosis, inflammation, tumor progression and malignant transformation. It is expected that exosomes will be important in various fields, such as in the development of drug delivery systems.
We are interested in the interaction between virus-infected cells and nearby non-infected cells. When virally infected cells are mixed and cultured with non-infected cells, fluorescent immunostaining can detect viral proteins in non-infected cells, appearing as punctate signals. From this observation, I became interested in exosomes as mediators of intercellular communication.
Comparison of exosomes recovered from various sources and under different conditions is challenging because of the lengthy and difficult methods involved, classically including ultracentrifugation. Therefore, from the viewpoint of operation ease (anybody can purify), purification time (purification can be done in a short time), and application versatility (exosomes themselves, as well as included proteins and nucleic acids can be analyzed), we explored commercially available exosome purification kits and found CosmoBio USA’s “ExoTrap™ Exosome Isolation Spin Column Kit” to be the ideal purification solution.
The greatest advantage of ExoTrap™ is that exosomes can be purified from small amounts of sample in "a short time" of only 30 minutes. As an exosome research beginner, ExoTrap™ allowed me to detect by western blot viral proteins associated with exosomes that could not be detected in the pre-purification culture supernatant. Also, I was able to show that viral proteins were not detected in ExoTrap™-purified exosomes recovered from culture supernatants of exosome inhibitor (GW4869)-treated cells(1). Although ExoTrap™ may not be suitable for inexpensively purifying large amounts of exosomes, it is highly recommended for rapid and easy purification of exosomes from small volumes.
Figures
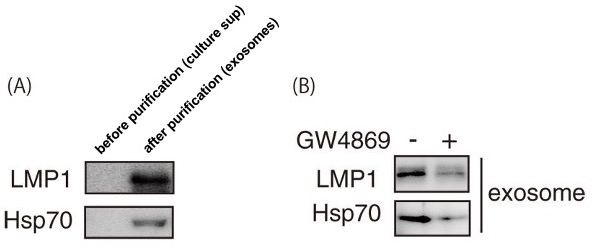 Figure 1. Western blot before and after exosome purification and before and after treatment with exosome inhibitor (A) Western blot showing LMP1 and Hsp70 expression in “ExoTrap™ Exosome Isolation Spin Column Kit”-purified exosomes. (B) Western blot showing LMP1 and Hsp70 expression in “ExoTrap™ Exosome Isolation Spin Column Kit”-purified exosomes from cell culture supernatants treated or not with exosome inhibitor (GW4869).
Figure 1. Western blot before and after exosome purification and before and after treatment with exosome inhibitor (A) Western blot showing LMP1 and Hsp70 expression in “ExoTrap™ Exosome Isolation Spin Column Kit”-purified exosomes. (B) Western blot showing LMP1 and Hsp70 expression in “ExoTrap™ Exosome Isolation Spin Column Kit”-purified exosomes from cell culture supernatants treated or not with exosome inhibitor (GW4869). 