Tear Mucin Assay Kit (O-Glycan Assay Method)
A kit to extract and fluorometrically determine the amount of mucin content in tear
Background
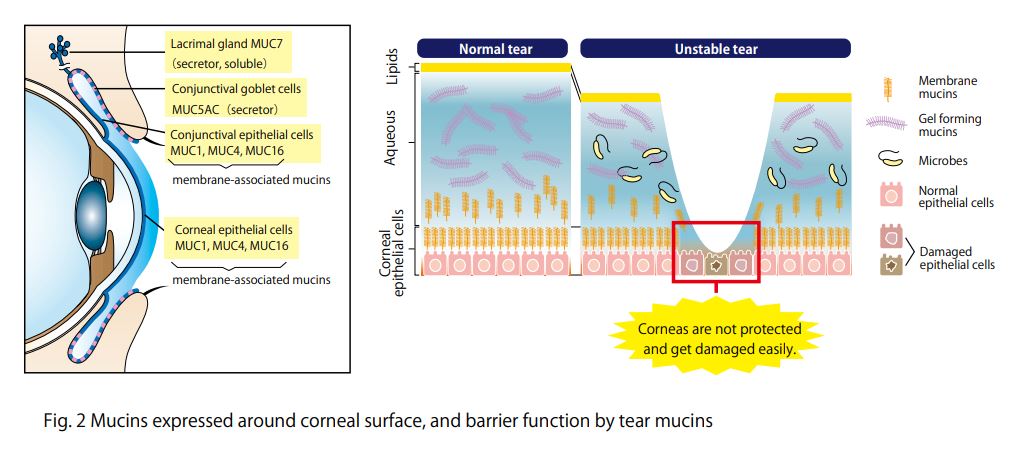
Mucins are major components in tear fluid and apical cell membranes on the ocular surface epithelia. Structurally, they are composed of tandem repeat domains containing heavily O-glycosylated serine and threonine residues. More than a half of its weight consists of O-glycans, which has hydrophilic nature (Fig. 1). The heavy glycosylation of mucins is believed to impart a highly negative charge and a hydrophilicity that provides a barrier to pathogen adherence and penetrance into the epithelium (Fig. 2). Alteration in both secreted and membrane-associated mucins occur in drying ocular surface diseases (Fig. 2). At the ocular surface, three types of mucins are present. The large gel-forming mucin MUC5AC is expressed by conjunctival goblet cells. Some cells of the lacrimal gland acini express the small soluble mucin MUC7. The corneal and conjunctival epithelia express the membrane-associated mucins MUCs 1, 4, and 16 (Fig. 2).

Features
- Under alkaline conditions, β-elimination of O-glycans from the core protein and simultaneous fluorescence labeling of the sugar chain reducing termini are performed, and the fluorescence intensity obtained is measured to measure the mucin in tear fluid.
- Schirmer test strips, which measure tear volume, can be used to measure tear mucin content.
- Please use it for the development of medicines and functional foods specialized for eyes such as dry eye, and ophthalmological research.
Application
For determination of mucin content in tear
Assay Principle
Mucins are family of high molecular (1000 kda-10000 kda) and heavily glycosylated protein. Mucin domains within the protein core are rich in threonine and serine. The reducing ends of sugar chain N-acetylgalactosamin (GalNAc) are linked to those amino acids by the post-translational O-glicosylation (Fig.1). Mucin content can be measured as reducing ends of sugar chain after β -eliminated by diluted alkali. Reducing ends of sugar chain react at high temperatures with 2-cyanoacetamide (2-CAN) to produce intensely fluorescent condensate.
Protocol
This product is designed for the measurement in fluorescence plate readers (96 well plates).
When using Fluorescence plate reader, it can measure 100 samples.
When you measure with a light spectrophotometer, please use it with a microcell.
Sample Preparation
- Collect a tear fluid for 5 minutes using Schirmer strips.
- If you store the filter paper collected tear fluid, please put it in micro test tube and store it at 4℃ . Avoid freezing.
Kit Components
Product number: MUC01 (for 50 samples)
- Extraction liquid: 30mL × 1 bottle
- Gel filtration carrier: 45mL x 1
- Standard solution (100 µg/mL N-acetylgalactosamine): 1.0 mL × 1 bottle
- Reagent A: 0.3 mL x 1 bottle
- Reagent B: 1.5 mL x 1 bottle
- Reaction stop solution: 15 mL × 1 bottle
- Empty column: 50 columns for 1 mL
- Centrifuge tubes: 50
Measurement of tear mucin
- Transfer the Schirmer strips into micro test tube and add 200 μL of Elution Buffer, and extract mucin for 1 hour at room temperature.
- During 1 hour extraction of step 1, prepare empty column. Set the empty column into centrifuge tube. Agitate the Slurry bottle, until uniformalized. Add 800 μL of uniformalized slurry into the empty column. Centrifuge the column for 10 minutes at 800 x g at room temperature. (Recommended equipment is a centrifugal of the swing rotor system.) Remove the pass though liquid in the bottom of centrifugal tube.
- Charge 50 μL of the extraction from Schirmer strips prepared at step 1 onto the upper part of the packed gel of step 2.
- Centrifuge the sample charged columns for 10 minutes at 800 x g at room temperature. Collect the pass through fraction (approximately 50 μL).
- Transfer the 20 uL of each pass through fraction (4) or standard solution( ①~⑧ ) into the another micro test tube (for 500 uL). Add 24 uL of reagent mixture (mix together Reagent A and Reagent B, 1:5 (v/v), just before to use) to the test tube. After mixing, heat the tube up to 100℃ for 30 minutes.
- Cool down the tube until room temperature, add the 200 uL of stop solution, and mix together with vortex mixer.
- Transfer the 100 uL of the solution into the wells of 96 well black plate, and then measure the fluorescence using fluorescence plate reader set at wavelength (Exitation:336 nm, Emission:383 nm).
- Create a standard curve by serial dilution as indicated in the below. Draw a smooth curve through these points to construct the calibration curve. Read the concentration of the sample from the calibration curve.
Usage Examples

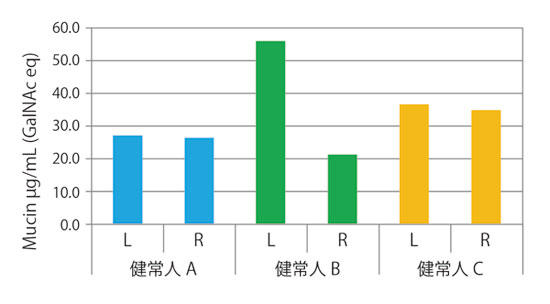
Reference data
The recommended measurement wavelengths are 336 nm for Ex and 383 nm for Em, but you might not be able to measure the fluorescence wavelength when fluorescence plate leader with interference filter system is used. In such a case, please shift the fluorescent wavelength to longer.


| Catalog Number | Product Name | Size |
| CSR-MUC01 | Tear Mucin Assay Kit (O-Glycan assay method) | 1 Kit |
Reference with product usage
- M. Yamada, C. Masaki, T. Mukaibo , T. Munemasa, T. Nodai, Y. Kondo, and R. Hosokawa. Altered Rheological Properties of Saliva with Aging in Mouse Sublingual Gland. (2022)Journal of Dental Research, 1-9
References
- Gipson IK. et al, Character of ocular surface mucins and their alteration in dry eye disease. Ocul Surf. 2004 Apr;2(2):131-48. PMID: 17216084
- Argueso P. et al, Decreased levels of the goblet cell mucin MUC5AC in tears of patients with Sjogren syndrome. Invest Ophthalmol Vis Sci. 2002 Apr;43(4):1004-11. PMID: 11923240
- Uchino Y. et al, Alteration of tear mucin 5AC in office workers using visual display terminals: The Osaka Study. JAMA Ophthalmol. 2014 Aug;132(8):985-92. doi: 10.1001/jamaophthalmol.2014.1008. PMID: 24903353
- Inatomi T. et al, Expression of secretory mucin genes by human conjunctival epithelia. Invest Ophthalmol Vis Sci. 1996 Jul;37(8):1684-92. PMID: 8675412
- Susumu Honda, Yoshikazu Matsuda, Masaye Takahashi, and Kazuaki Kakehi, Fluorimetric Determination of Reducing Carbohydrates with2-Cyanoacetamide and Application to Automated Analysis of Carbohydrates as Borate Complexes. (1980) Analytical Chemistry, Vol.52, No. 7
- Crowther RS. et al, Fluorometric assay of O-linked glycoproteins by reaction with 2-cyanoacetamide. Anal Biochem. 1987 May 15;163(1):170-4. PMID: 3619016
- Okazaki Y, Han Y, Kayahara M, Watanabe T, Arishige H, Kato N. Consumption of curcumin elevates fecal immunoglobulin A, an index of intestinal immune function, in rats fed a high-fat diet. J Nutr Sci Vitaminol (2010); 56(1): 68-71.
- Yukako Okazaki, Hiroyuki Tomotake, Kazuhisa Tsujimoto, Masahiro Sasaki,and Norihisa Kato. Consumption of a Resistant Protein, Sericin, Elevates Fecal Immunoglobulin A, Mucins, and Cecal Organic Acids in Rats Fed a High-Fat Diet. (2011) The Journal of Nutrition , 21, 10.3945/ jn.111.144246.
- Zaki Utama & Yukako Okazaki & Hiroyuki Tomotake & Norihisa Kato, Tempe Consumption Modulates Fecal Secondary Bile Acids, Mucins, Immunoglobulin A, Enzyme Activities, and Cecal Microflora and Organic Acids in Rats. Plant Foods Hum Nutr (2013) 68: 177–183
