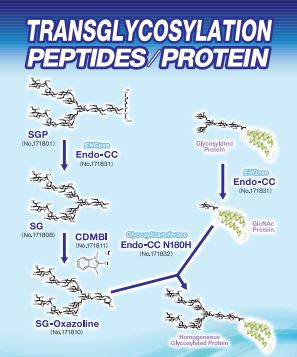
Product Lineup
ENGase
Brochures / Flyers
Chemical modification
of glyco-protein
View PDF
Conjugation reaction
of glycan with amine
View PDF
Fushimi Pharmaceuticals Website (external site)
http://www.fushimi.co.jp/en/industrial-chemicals/industrial-chemicals-06.html
Fushimi Pharmaceuticals (FSP) Products Dashboard
Glycan-related products - Industrial Chemicals
Product Lineup
|
|
ENGase |
|
All Products
| Catalog Number | Product Name | Size | Structure |
| FSP-171801 | Sialylglycopeptide (SGP) ■Formula,Molecular Weight: It is well known that the synthesis of structurally homogeneous carbohydrate chain is difficult due to various types of glycoside bonding. On the other hand, egg yolk is known to contain a glycopeptide with homogeneous structure. The main component is SGP. Fushimi Pharmaceutical Co. has succeeded in producing chicken egg SGP having homogeneous structure in a large quantity at low production cost. Their hope is this will open various opportunities to develop new bio-pharmaceuticals and bio-anallytical reagents. |
10 mg |  |
| FSP-171803 | alpha 2,3-Sialylglycopeptide (alpha 2,3-SGP) ■Formula,Molecular Weight: C112H189N15O70, 2,865.76 ■Assay:min. 95% (HPLC) |
1 mg |  |
| FSP-171804 | Asialoglycopeptide (Asialo-SGP) ■Formula,Molecular Weight: |
1 mg | 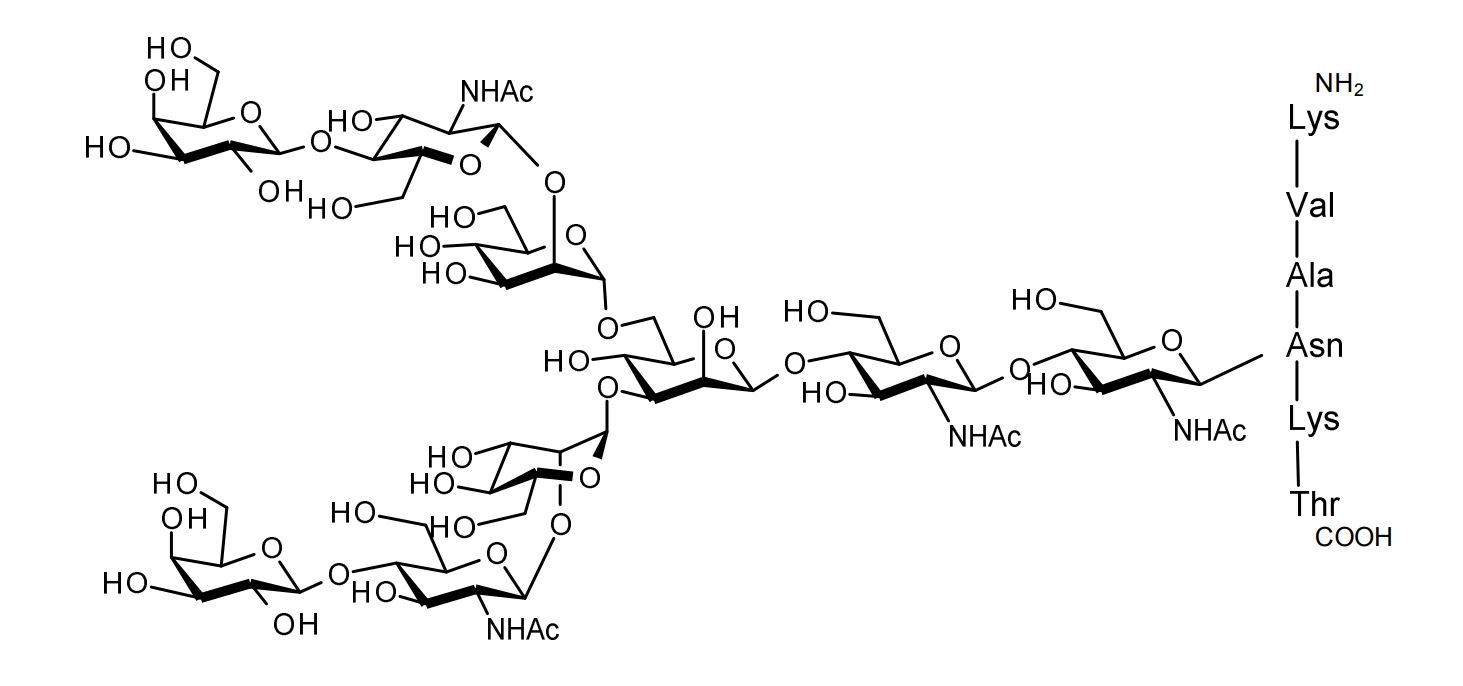 |
| FSP-171816 | Agalactoglycopeptide (Agalacto-SGP) ■Formula,Molecular Weight: |
1 mg | 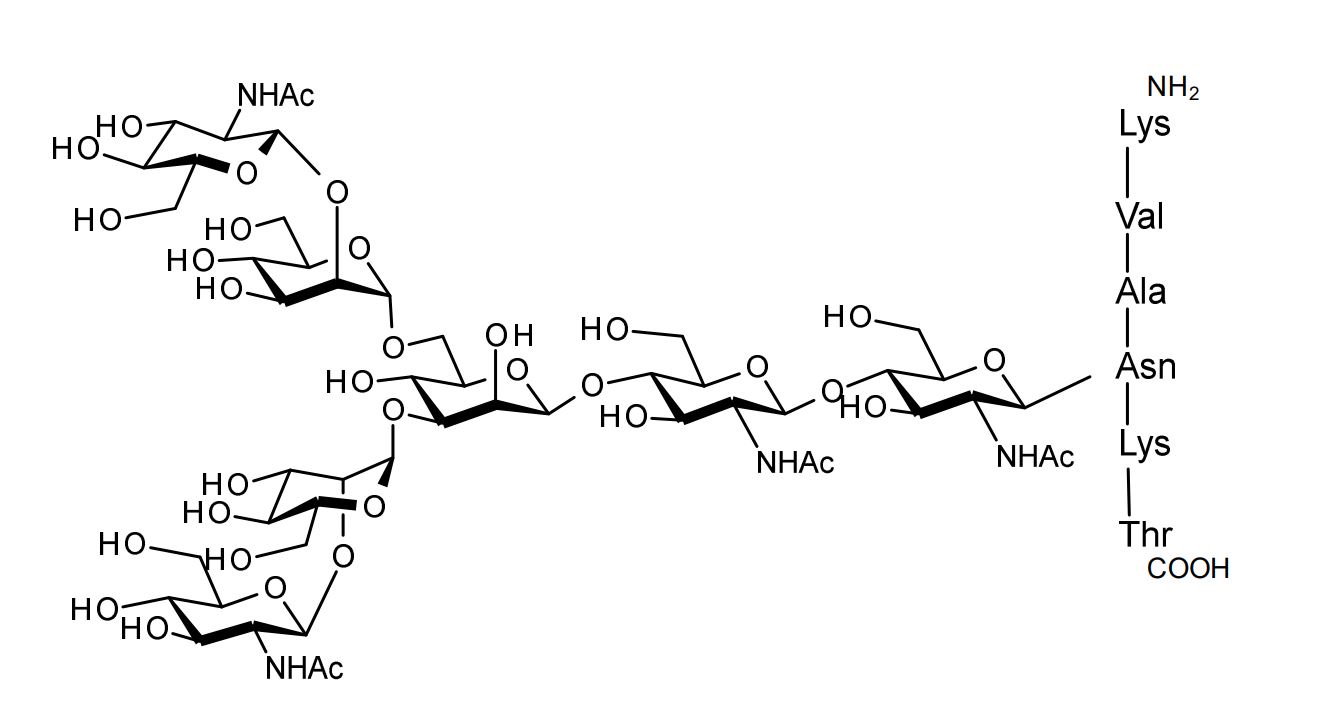 |
| Catalog Number | Product Name | Size | Structure |
| FSP-171808 | alpha 2,6-Sialylglycan (alpha 2,6-SG) ■Formula,Molecular Weight: |
10 mg | 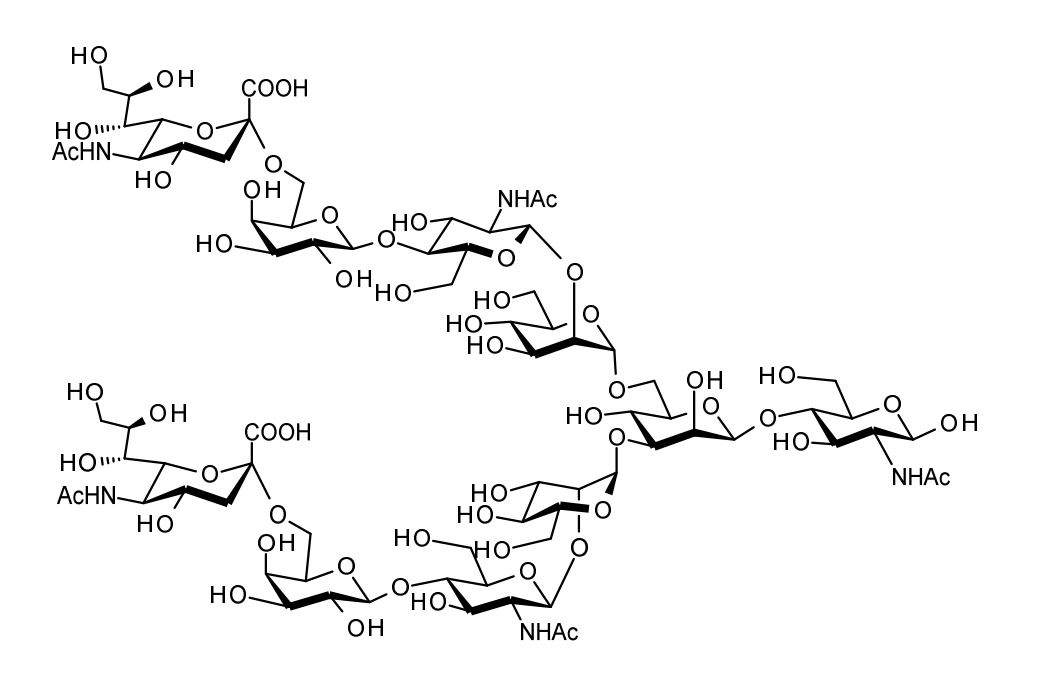 |
| FSP-171817 | Asialoglycan (G2-Glycan) ■Formula,Molecular Weight: |
1 mg | 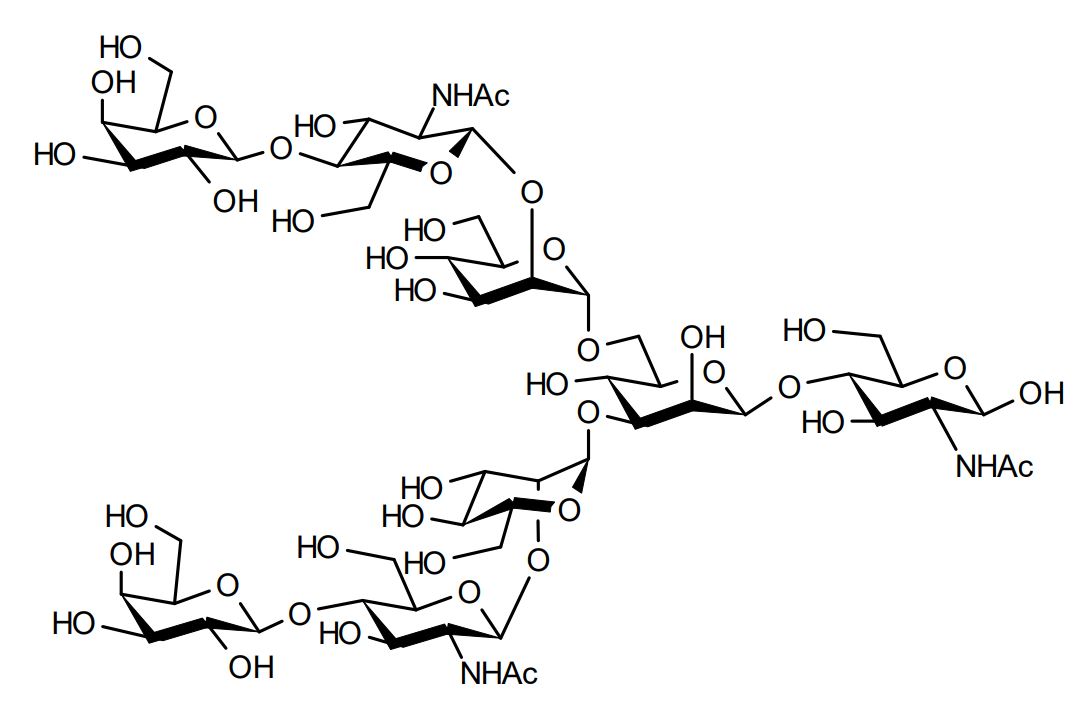 |
| FSP-171818 | Agalactoglycan (G0-Glycan) ■Formula,Molecular Weight: |
1 mg | 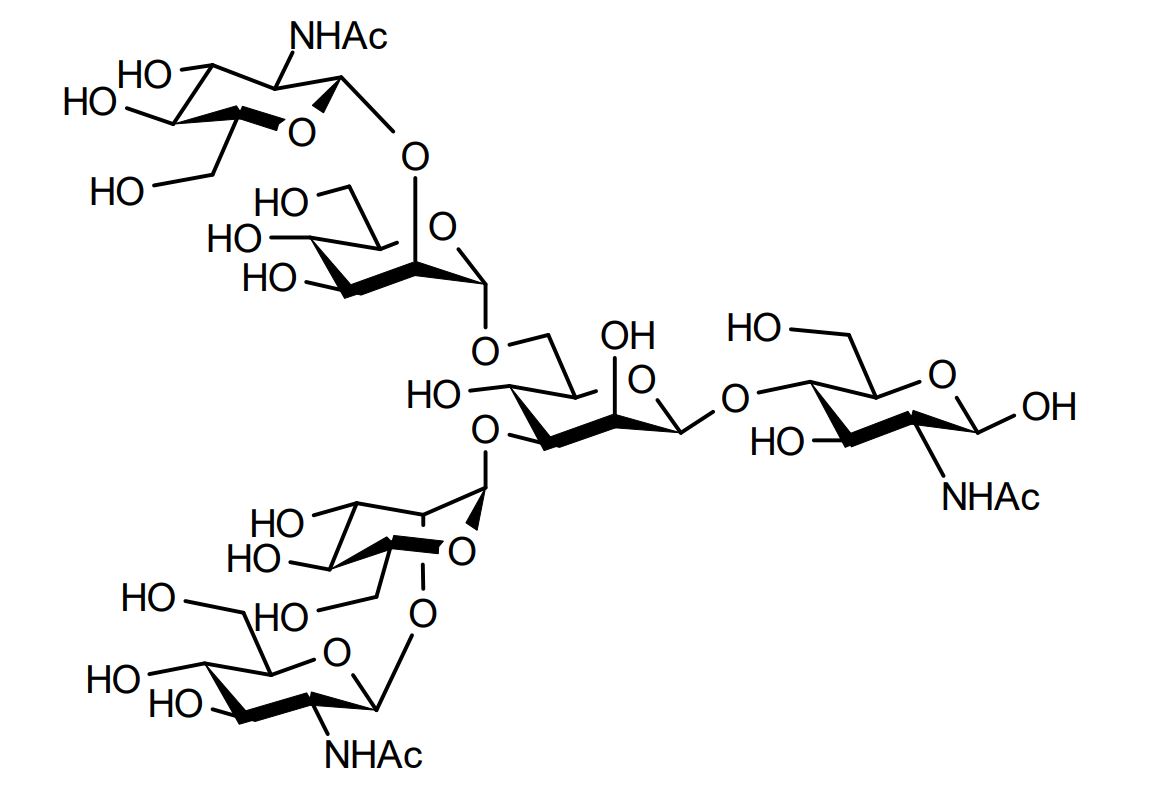 |
| FSP-171810 | Sialyglycan-Oxazoline (SG-Oxazoline (SG-Oxa)) ■Formula,Molecular Weight: |
1 mg | 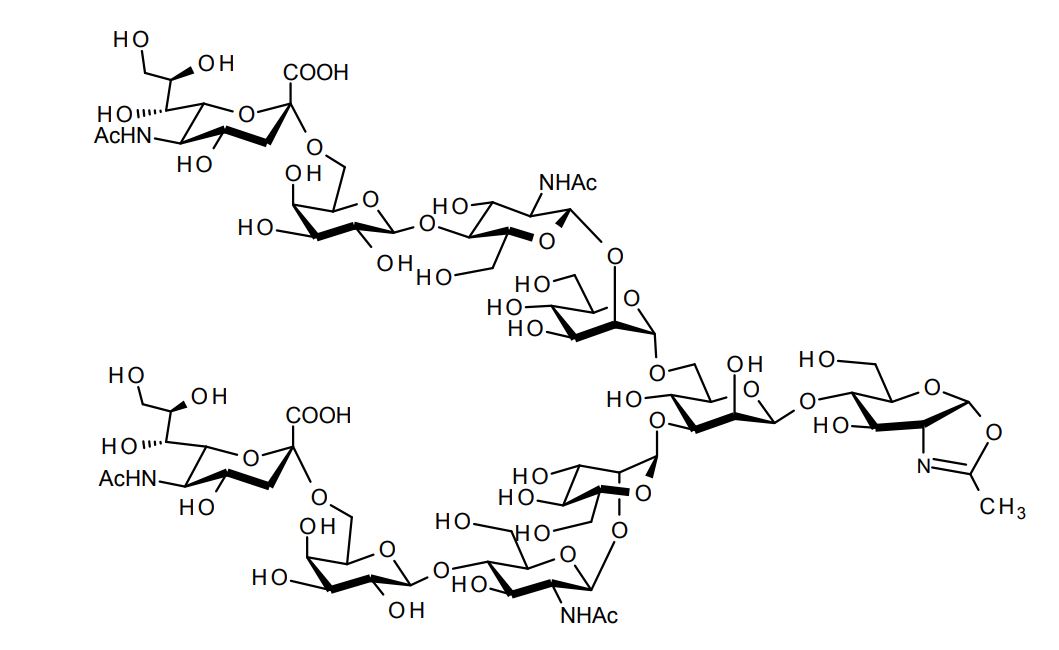 |
| FSP-171814 | Asialoglycan-Oxazoline (G2-Oxazoline (G2-Oxa), Asialo-Oxazoline) ■Formula,Molecular Weight: |
1 mg | 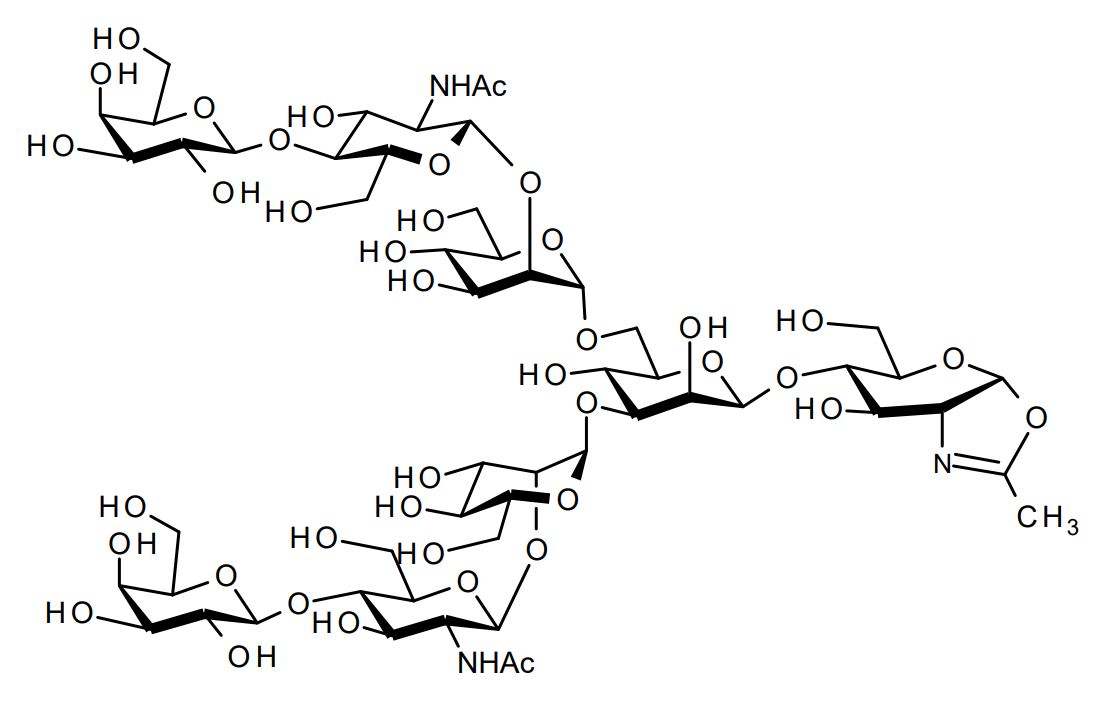 |
| FSP-171815 | Agalactoglycan-Oxazoline (G0-Oxazoline (G0-Oxa), Agalacto-Oxazoline) ■Formula,Molecular Weight: |
1 mg | 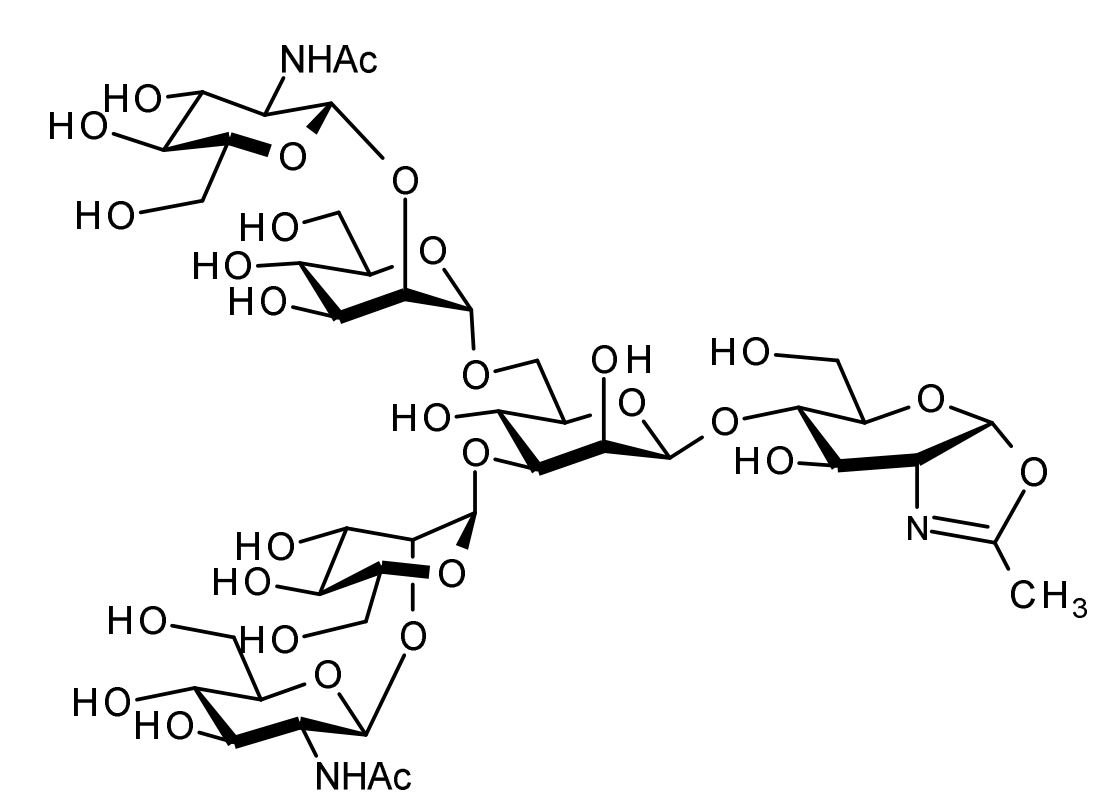 |
ENGase
| Catalog Number | Product Name | Size |
| FSP-171831 | Endo-CC (Wild Type) ■Source:Endo-CC recombinant from Coprinopsis cinerea is expressed in E.coli as a fusion to His tag. |
300 munits |
| FSP-171832 | Endo-CC N180H (Mutant) ■Source:Endo-CC N180H recombinant from Coprinopsis cinerea is expressed in E.coli as a fusion to His tag. |
300 munits |
| Catalog Number | Product Name | Size | Structure |
| FSP-171811 | CDMBI ■Product Name:CDMBI |
1 g |  |
| Catalog Number | Product Name | Size | Structure |
| FSP-171813 | Fmoc-Asn-4Sialylglycan (Fmoc-4SGN) | 1 mg | 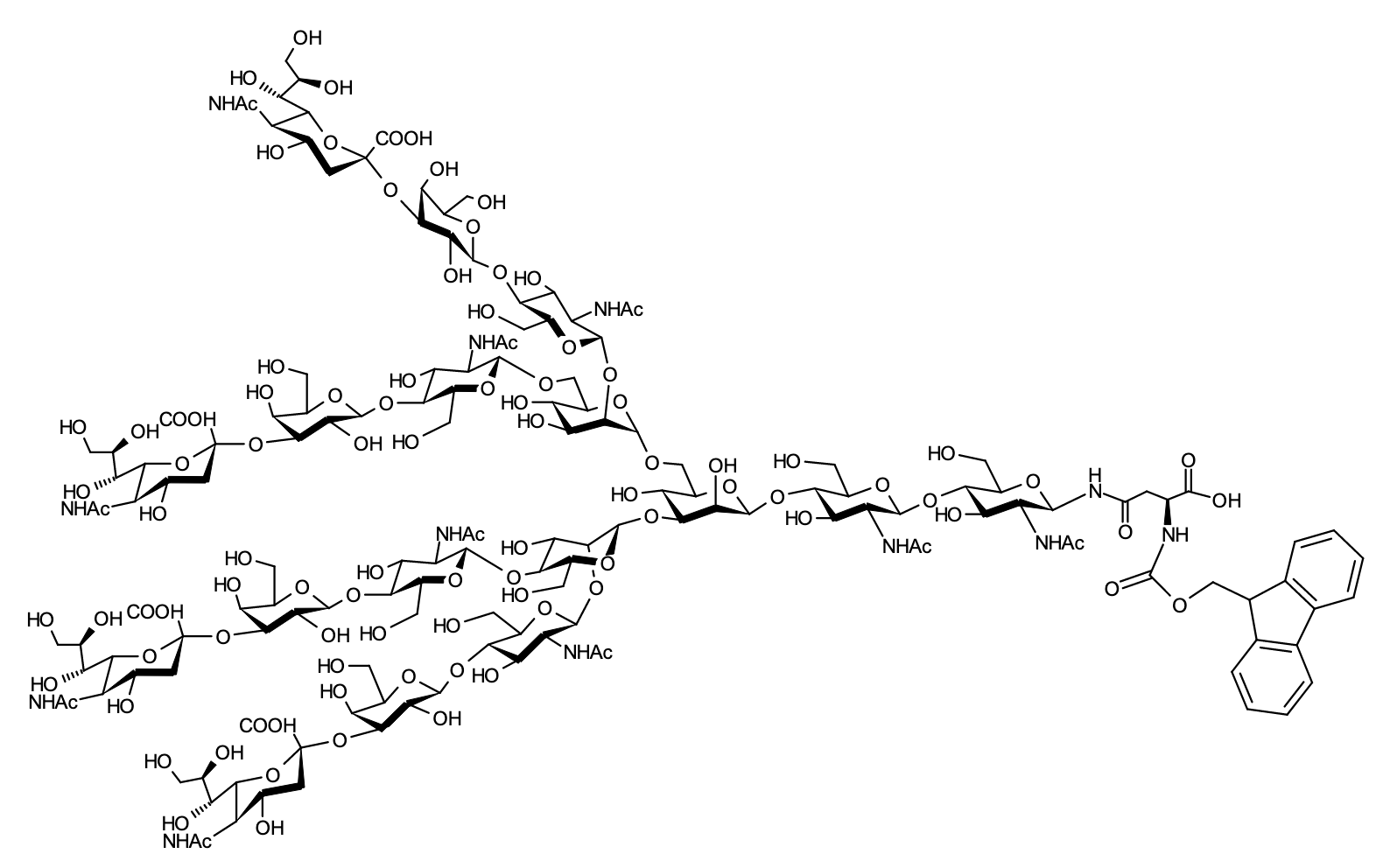 |
Publications
1) Ashida H, Fujimoto T, Kurihara S, Nakamura M, Komeno M, Huang Y, Katayama T, Kinoshita T, Takegawa K:
1,6-α-L-Fucosidases from Bifidobacterium longum subsp. infantis ATCC 15697 involved in the degradation of core-fucosylated N-glycan.
Journal of Applied Glycoscience 67, 23-29 (2020)
DOI:10.5458/jag.jag.JAG-2019_0016
2) Seki H, Huang Y, Arakawa T, Yamada C, Kinoshita T, Iwamoto S, Higuchi Y, Takegawa K, Fushinobu S:
Structural basis for the specific cleavage of core-fucosylated N-glycans by endo-β-N-acetylglucosaminidase from the fungus Cordyceps militaris.
J Biol Chem. 294, 17143-17154 (2019)
PMID:31548313 DOI:10.1074/jbc.RA119.010842
3) Manabe S, Yamaguchi Y, Matsumoto K, Fuchigami H, Kawase T, Hirose K, Mitani A, Sumiyoshi W, Kinoshita T, Abe J, Yasunaga M, Matsumura Y, Ito Y:
Characterization of antibody products obtained through enzymatic and nonenzymatic glycosylation reactions with a glycan oxazoline and preparation of a homogeneous antibody-drug conjugate via Fc N-glycan.
Bioconjug Chem.30, 1343-1355 (2019)
PMID:30938513 DOI:10.1021/acs.bioconjchem.9b00132
4) Huang Y, Higuchi Y, Kinoshita T, Mitani A, Eshima Y, Takegawa K:
Characterization of novel endo-β-N-acetylglucosaminidases from Sphingobacterium species, Beauveria bassiana and Cordyceps militaris that specifically hydrolyze fucose-containing oligosaccharides and human IgG.
Sci. Rep. 8, 246 (2018)
PMID:29321565 DOI:10.1038/s41598-017-17467-y.
5) Higuchi Y, Eshima Y, Huang Y, Kinoshita T, Sumiyoshi W, Nakakita S, Takegawa K:
Highly efficient transglycosylation of sialo-complex-type oligosaccharide using Coprinopsis cinerea endoglycosidase and sugar oxazoline.
Biotechnol. Lett.39, 157-162 (2017)
PMID:27714557 DOI:10.1007/s10529-016-2230-0
6) Suda M, Sumiyoshi W, Kinoshita T, Ohno S:
Reaction of sugar oxazolines with primary amines
Tetrahedron Letters 57, 5446–5448 (2016)
DOI:10.1016/j.tetlet.2016.10.074
7) Eshima Y, Higuchi Y, Kinoshita T, Nakakita S, Takegawa K:
Transglycosylation activity of glycosynthase mutants of endo-β-N-acetylglucosaminidase from Coprinopsis cinerea.
PLoS ONE 10, e0132859 (2015)
PMID:26197478 DOI:10.1371/journal.pone.0132859