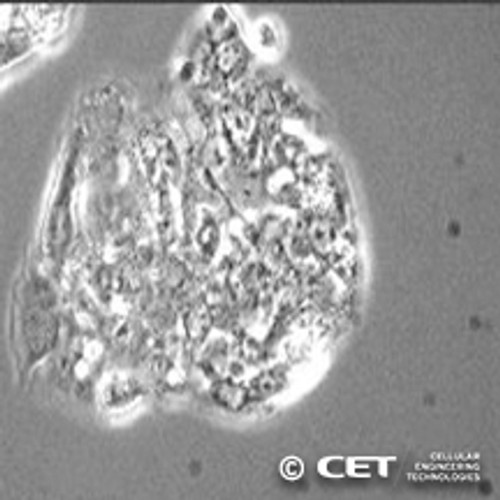
Human HepG2 Hepatocellular Carcinoma Cells
Cellular Engineering Technologies
- SKU:
- CET-CR1015-500
- Shipping:
- Calculated at Checkout
HepG2 Hepatocellular Carcinoma Cells
HepG2 Hepatocellular Caincoma Cells are a widely used human hepatocellular carcinoma (HCC) cell line derived from a liver biopsy of a 14-year-old male donor. These cells serve as a critical model system for studying liver function, cancer biology, drug metabolism, and hepatotoxicity [i]. With their ability to produce major plasma proteins, HepG2 cells provide a robust platform for analyzing liver-specific processes, including metabolism, protein secretion, and tumor progression.
HepG2 cells are commonly used to investigate various aspects of liver disease, including carcinogenesis, tumor metastasis, and cytotoxic responses to drugs and environmental toxins. Moreover, these cells secrete essential plasma proteins such as albumin, α2-macroglobulin, α1-anti-trypsin, and plasminogen, making them ideal for protein-based assays, molecular biology applications, and biochemical studies. As a result, their versatility enables research into key hepatic functions, including lipid metabolism, glucose regulation, and drug detoxification.
As an immortalized hepatic cell line, HepG2 cells offer a homogeneous and reproducible alternative to primary hepatocytes, eliminating the need for liver biopsies. They exhibit a characteristic growth pattern, initially proliferating slowly and forming clusters rather than a traditional monolayer. Their adaptability and well-documented behavior make them an invaluable resource for studying liver disease mechanisms, pharmaceutical development, and toxicology assessments.
Product Specifications
- Source: Liver biopsy from a 14-year-old male donor
- Cell Type: Human hepatocellular carcinoma cell line
- Secretion Profile: Albumin, α2-macroglobulin, α1-anti-trypsin, plasminogen
- Applications:
- Cancer research and drug discovery
- Liver metabolism and toxicology studies
- Signaling pathway analysis
- Protein expression and molecular biology research
- Spheroid genotoxicity testing [ii]
- Growth Characteristics: Cluster-forming with slow initial proliferation
Recommended Products for HepG2 Cells
- HepG2 Hepatocellular Carcinoma Expansion Media (CET-MR1010)
- Human HepG2 Research Starter Kit (CET-BR1003)
Shipping & Storage
- Vial Size: Approximately 500,000 cells
- Shipping Conditions: Shipped on dry ice to maintain viability
- Storage Recommendation: Store in liquid nitrogen vapor phase for long-term preservation
HepG2 cells continue to be a cornerstone in liver disease research, offering a reliable and well-characterized model for studying hepatocellular carcinoma, drug metabolism, and liver toxicity. Their ability to produce key liver proteins and respond to environmental and pharmaceutical agents makes them indispensable for academic and commercial research.
| Documents & Links for Human HepG2 Hepatocellular Carcinoma Cells | |
| Datasheet | Human HepG2 Hepatocellular Carcinoma Cells Datasheet |
| Vendor Page | Human HepG2 Hepatocellular Carcinoma Cells at Cellular Engineering Technologies |
| Documents & Links for Human HepG2 Hepatocellular Carcinoma Cells | |
| Datasheet | Human HepG2 Hepatocellular Carcinoma Cells Datasheet |
| Vendor Page | Human HepG2 Hepatocellular Carcinoma Cells |
| Citations for Human HepG2 Hepatocellular Carcinoma Cells – 20 Found |
| Udagawa, Daisuke; Nagata, Shogo; Yagi, Hiroshi; Nishi, Kotaro; Morisaku, Toshinori; Adachi, Shungo; Nakano, Yutaka; Tanaka, Masayuki; Hori, Shutaro; Hasegawa, Yasushi; Abe, Yuta; Kitago, Minoru; Kitagawa, Yuko. A Novel Approach to Orthotopic Hepatocyte Transplantation Engineered With Liver Hydrogel for Fibrotic Livers, Enhancing Cell-Cell Interaction and Angiogenesis. Cell Transplantation. 2024;33( 38770981):9636897241253700. PubMed |
| Sasaki, Kazuki; Akagi, Takami; Asaoka, Tadafumi; Eguchi, Hidetoshi; Fukuda, Yasunari; Iwagami, Yoshifumi; Yamada, Daisaku; Noda, Takehiro; Wada, Hiroshi; Gotoh, Kunihito; Kawamoto, Koichi; Doki, Yuichiro; Mori, Masaki; Akashi, Mitsuru. Construction of three-dimensional vascularized functional human liver tissue using a layer-by-layer cell coating technique. Biomaterials. 2017;133:263-274. PubMed |
| Ichimaru, Yoshimi; Fujii, Takeshi; Saito, Hiroaki; Sano, Makoto; Uchiyama, Taketo; Miyairi, Shinichi. 5-Bromoindirubin 3'-(O-oxiran-2-ylmethyl)oxime: A long-acting anticancer agent and a suicide inhibitor for epoxide hydrolase. Bioorganic & Medicinal Chemistry. 2017;25(17):4665-4676. PubMed |
| Asai, Akira; Tsuchimoto, Yusuke; Ohama, Hideko; Fukunishi, Shinya; Tsuda, Yasuhiro; Kobayashi, Makiko; Higuchi, Kazuhide; Suzuki, Fujio. Host antitumor resistance improved by the macrophage polarization in a chimera model of patients with HCC. Oncoimmunology. 6(4):e1299301. PubMed |
| Yoshikawa, Chiaki; Sakakibara, Keita; Nakaji-Hirabayashi, Tadashi; Yamazaki, Tomohiko; Tsujii, Yoshinobu. Well-defined monolith morphology regulates cell adhesion and its functions. Materials Science & Engineering. C, Materials For Biological Applications. 2019;105:110108. PubMed |
| Chauhan, Ranjit; Shimizu, Yoshimi; Watashi, Koichi; Wakita, Takaji; Fukasawa, Masayoshi; Michalak, Tomasz I. Retrotransposon elements among initial sites of hepatitis B virus integration into human genome in the HepG2-NTCP cell infection model. Cancer Genetics. 2019;235-236:39-56. PubMed |
| Nashimoto, Yuji; Okada, Ryu; Hanada, Sanshiro; Arima, Yuichiro; Nishiyama, Koichi; Miura, Takashi; Yokokawa, Ryuji. Vascularized cancer on a chip: The effect of perfusion on growth and drug delivery of tumor spheroid. Biomaterials. 2020;229:119547. PubMed |
| Masuda, Atsutaka; Nakamura, Toru; Abe, Mitsuhiko; Iwamoto, Hideki; Sakaue, Takahiko; Tanaka, Toshimitsu; Suzuki, Hiroyuki; Koga, Hironori; Torimura, Takuji. Promotion of liver regeneration and anti‑fibrotic effects of the TGF‑β receptor kinase inhibitor galunisertib in CCl4‑treated mice. International Journal Of Molecular Medicine. 2020;46(1):427-438. PubMed |
| Asai, Akira; Yasuoka, Hidetaka; Matsui, Masahiro; Tsuchimoto, Yusuke; Fukunishi, Shinya; Higuchi, Kazuhide. Programmed Death 1 Ligand Expression in the Monocytes of Patients with Hepatocellular Carcinoma Depends on Tumor Progression. Cancers. 2020;12(8) PubMed |
| Matsubayashi, Masaya; Sakaguchi, Yoshihiko M; Sahara, Yoshiki; Nanaura, Hitoki; Kikuchi, Sotaro; Asghari, Arvand; Bui, Linh; Kobashigawa, Shinko; Nakanishi, Mari; Nagata, Riko; Matsui, Takeshi K; Kashino, Genro; Hasegawa, Masatoshi; Takasawa, Shin; Eriguchi, Masahiro; Tsuruya, Kazuhiko; Nagamori, Shushi; Sugie, Kazuma; Nakagawa, Takahiko; Takasato, Minoru; Umetani, Michihisa; Mori, Eiichiro. 27-Hydroxycholesterol regulates human SLC22A12 gene expression through estrogen receptor action. Faseb Journal : Official Publication Of The Federation Of American Societies For Experimental Biology. 2021;35(1):e21262. PubMed |
| Hara, Yuichi; Yanatori, Izumi; Tanaka, Atsushi; Kishi, Fumio; Lemasters, John J; Nishina, Sohji; Sasaki, Kyo; Hino, Keisuke. Iron loss triggers mitophagy through induction of mitochondrial ferritin. Embo Reports. 2020;21(11):e50202. PubMed |
| Iwasa, Naoki; Matsui, Takeshi K; Iguchi, Naohiko; Kinugawa, Kaoru; Morikawa, Naritaka; Sakaguchi, Yoshihiko M; Shiota, Tomo; Kobashigawa, Shinko; Nakanishi, Mari; Matsubayashi, Masaya; Nagata, Riko; Kikuchi, Sotaro; Tanaka, Tatsuhide; Eura, Nobuyuki; Kiriyama, Takao; Izumi, Tesseki; Saito, Kozue; Kataoka, Hiroshi; Saito, Yuichi; Kimura, Wataru; Wanaka, Akio; Nishimura, Yuhei; Mori, Eiichiro; Sugie, Kazuma. Gene Expression Profiles of Human Cerebral Organoids Identify PPAR Pathway and PKM2 as Key Markers for Oxygen-Glucose Deprivation and Reoxygenation. Frontiers In Cellular Neuroscience. 15:605030. PubMed |
| Tani, Naoto; Ikeda, Tomoya; Ishikawa, Takaki. Relationship between clock gene expression and CYP2C19 and CYP3A4 with benzodiazepines. Human & Experimental Toxicology. 2023;42:9603271231171643. PubMed |
| Tani, Naoto; Ikeda, Tomoya; Ishikawa, Takaki. Effect of methamphetamine on clock genes and drug-metabolizing enzyme expression. Human & Experimental Toxicology. 2022;41:9603271221124092. PubMed |
| Kimura, Takefumi; Iwadare, Takanobu; Wakabayashi, Shun-Ichi; Kuldeep, Seema; Nakajima, Tomoyuki; Yamazaki, Tomoo; Aomura, Daiki; Zafar, Hamim; Iwaya, Mai; Joshita, Satoru; Uehara, Takeshi; Pydi, Sai P; Tanaka, Naoki; Umemura, Takeji. Thrombospondin 2 is a key determinant of fibrogenesis in non-alcoholic fatty liver disease. Liver International : Official Journal Of The International Association For The Study Of The Liver. 2024;44(2):483-496. PubMed |
| Udagawa, Daisuke; Nagata, Shogo; Yagi, Hiroshi; Nishi, Kotaro; Morisaku, Toshinori; Adachi, Shungo; Nakano, Yutaka; Tanaka, Masayuki; Hori, Shutaro; Hasegawa, Yasushi; Abe, Yuta; Kitago, Minoru; Kitagawa, Yuko. A Novel Approach to Orthotopic Hepatocyte Transplantation Engineered With Liver Hydrogel for Fibrotic Livers, Enhancing Cell-Cell Interaction and Angiogenesis. Cell Transplantation. 2024;33:9636897241253700. PubMed |
| Minami, Tatsuro; Satoh, Kimio; Nogi, Masamichi; Kudo, Shun; Miyata, Satoshi; Tanaka, Shin-ichi; Shimokawa, Hiroaki. Statins up-regulate SmgGDS through β1-integrin/Akt1 pathway in endothelial cells. Cardiovascular Research. 2016;109(1):151-61. PubMed |
| Yasuoka, Hidetaka; Asai, Akira; Ohama, Hideko; Tsuchimoto, Yusuke; Fukunishi, Shinya; Higuchi, Kazuhide. Increased both PD-L1 and PD-L2 expressions on monocytes of patients with hepatocellular carcinoma was associated with a poor prognosis. Scientific Reports. 2020;10(1):10377. PubMed |
| Tani, Naoto; Ikeda, Tomoya; Ishikawa, Takaki. Relationship between clock gene expression and CYP2C19 and CYP3A4 with benzodiazepines. Human & Experimental Toxicology. 2023;42:9603271231171643. PubMed |
| Tani, Naoto; Ikeda, Tomoya; Ishikawa, Takaki. Effect of methamphetamine on clock genes and drug-metabolizing enzyme expression. Human & Experimental Toxicology. 2022;41:9603271221124092. PubMed |