AteloCell®3D Collagen Scaffold
AteloCell®3D Collagen Scaffold
Koken Dashboard Page
In Vitro Applications |
What is Atelocollagen?
Collagen is an extracellular matrix found in the dermis, ligaments, bones, etc., and accounts for approximately 30% of the total protein in the human body. The most abundant type of collagen is type I collagen, which has a molecular weight of approximately 300 kDa and comprises three polypeptides. Type I collagen occurs as a trimer with a central right-handed triple helix region flanked by N-terminal and C-terminal non-helical telopeptide regions. These telopeptide regions are composed of two α1 chains and one α2 chain. The triple helix region is conserved among species and shows low immunogenicity, while the telopeptide regions exhibit high immunogenicity. Removal of the telopeptide regions by protease treatment produces atelocollagen, which retains the same properties as collagen. Unwinding of the triple helix of collagen and atelocollagen by heat degeneration produces gelatin. Gelatin is a random coil single polypeptide and has high immunogenicity. Peptides obtained by hydrolysis with strong acids, strong alkalis, or by enzymatic treatment are called hydrolysed collagen. Gelatin and hydrolysed collagen have totally different properties from collagen due to their structural differences compared to collagen.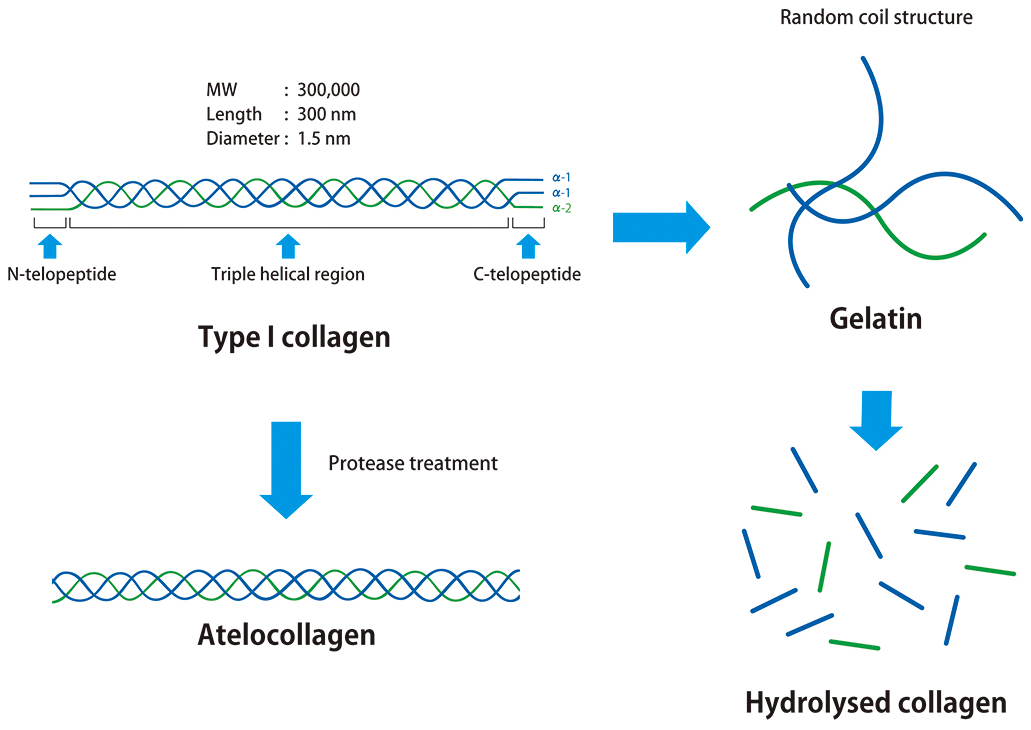
Does collagen coating influence experimental results?
Collagen and cell culture
Collagen is widely used as a coating for cell cultureware to promote cell adhesion and proliferation. The coating potential of collagen was first reported in a 1956 paper by Gey et al. who established the world’s first human cell line called HeLa in 1951. Essentially, after the preparation of a disk-like collagen gel with a thickness of about 3.8 mm and a diameter of about 3.8 cm, some moisture is removed using a glass roller bottle. Thereafter, 29 cell lines and tissues including 24 human-derived samples were cultured on the gel. This led to the enhanced proliferation of 28 samples in comparison to culture on glass, but frog skin tissue showed no change. In the addendum of the same paper, they describe the direct addition of collagen solution into a roller bottle to prepare a dry collagen gel; this is similar to current collagen coating methods. (Ehrmann RL, Gey GO. The growth of cells on a transparent gel of reconstituted rat-tail collagen. J Natl Cancer Inst. 1956 Jun;16(6):1375-403)
Stability of collagen following cell cultureware coating
Collagen is commonly used for coating in cell culture and various brands of collagen pre-coated cultureware are sold in order to reduce research labour. However, it has been reported that collagen oxidises when exposed to air, forming intramolecular cross-links. Nadzir et al. observed the fibrous structure of collagen after storage of collagen-coated cell cultureware in nitrogen versus atmospheric air. They observed normal collagen fibers in the cell cultureware stored under nitrogen, whereas the fiber structures of collagen in cell cultureware kept under atmosphere air changed into a granular form. In addition, we compared cell function after primary hepatocytes were cultured on collagen-coated plates prepared in advance or purchased commercially and found that commercially coated plates showed similar results to gelatin, suggesting that collagen in pre-coated commercial cultureware may have been thermally denatured.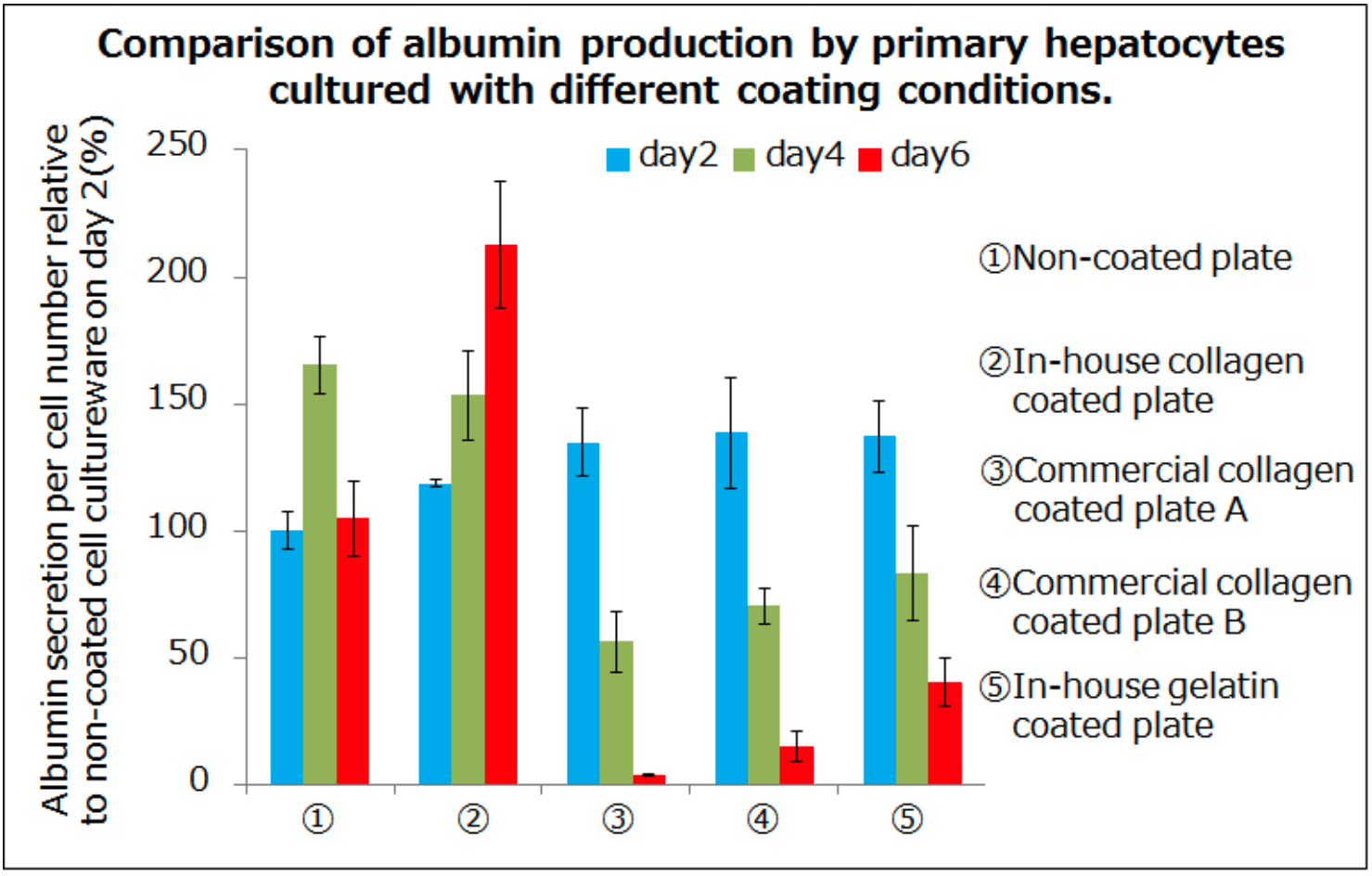
Koken in-house data
Various collagen coating methods
So, what method is most optimal for coating cell cultureware with collagen? From our in-house research, we found approximately ten good protocols created by collagen makers, cell cultureware makers and reference books; they were narrowed down to the following methods described below: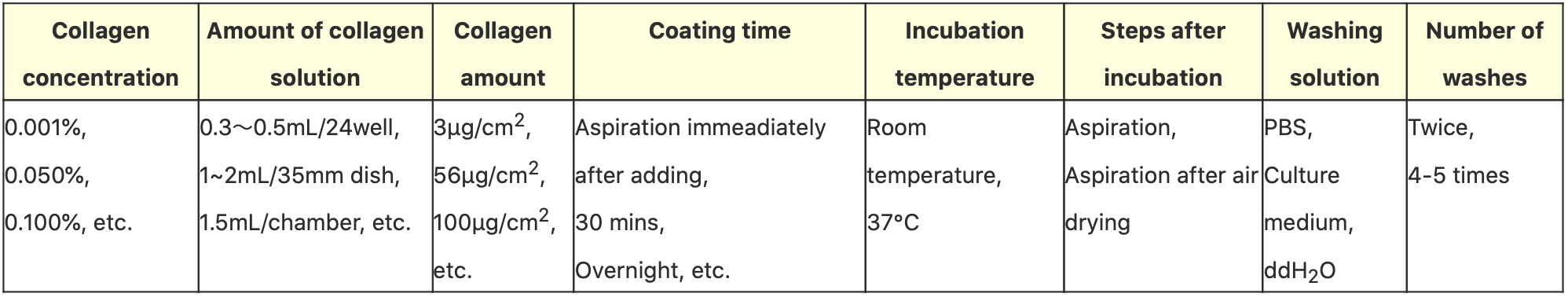
Note: The table above was produced on the basis of publicly available information, so the appropriate conditions may differ depending on the cell cultureware and cells used.
Koken recommends a collagen coating method in which a 0.3% or 0.5% collagen acidic solution is diluted about 10 fold and then dispensed to approximately 50μg/cm2 of cultureware. For those not familiar with collagen coating, we recommend evaluation of a range of collagen concentrations through the use of a dilution series.
Comparison between collagen coating and collagen gel
In the pioneering study by Gey et al cell culture was performed on collagen gels. This raised the question if there is any difference in the behavior of cells cultured on collagen gels versus collagen-coated cell cultureware. We compared the two methods in HepG2 cell culture. The expression of liver function-related genes in cells cultured on collagen gels was enhanced compared to cells cultured on collagen-coated cell cultureware. In recent years, reports have proliferated relating to the significance of cell cultureware hardness in cell adherence and cell function. It is likely that substrate hardness is a factor underlying the different performance of cells between the two methods.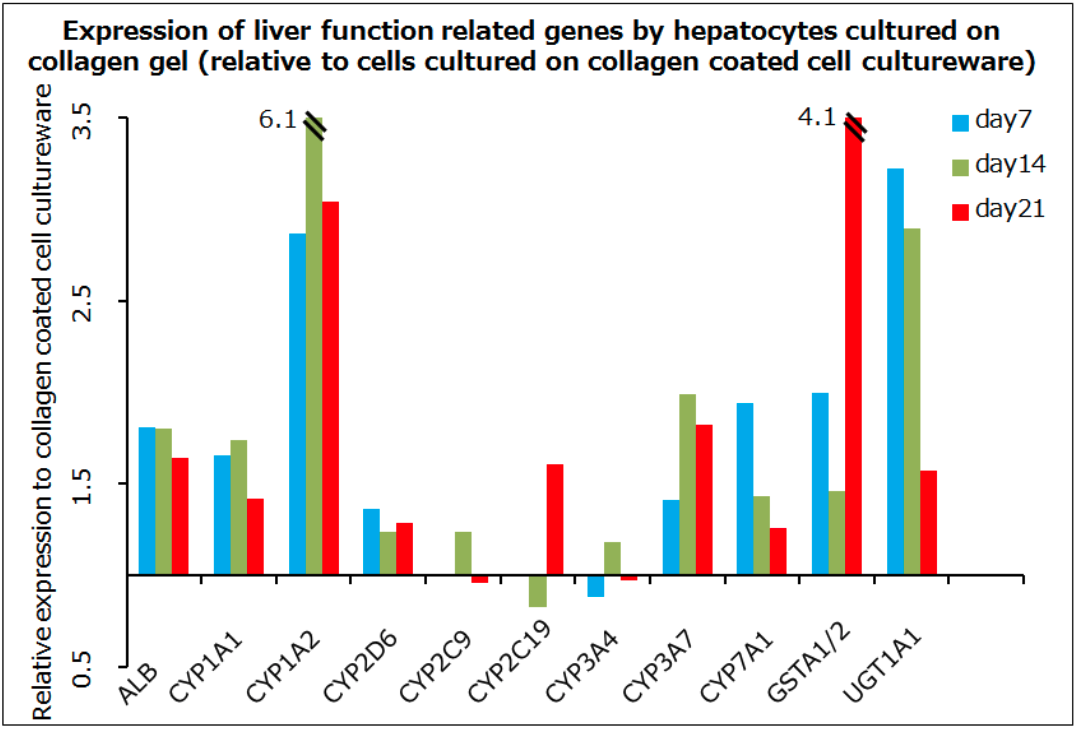
Koken in-house data
Connecting in vitro experiments to in vivo experiments with 3D culture
3D culture is attracting a variety of researchers
Many researchers have become interested in 3D culture. Until recently, most scientists viewed 3D culture as something utilized only in regenerative medicine research. However, in the past few years, cancer researchers have increasingly adopted 3D culture. One of the reasons for this gap between image and reality is the unexpected lack of effective antitumor properties of candidate drugs in animal models, a result that contrasts with the strong antitumor effects observed for these candidates in plate culture. Apparently, standard plate culture does not realistically reflect the actual tumour microenvironment. 3D culture is expected to become more common because researchers are turning to 3D culture in order to perform in vitro experiments under conditions that better mimic in vivo conditions.
3D culture with collagen gel
Collagen accounts for approximately 30% of the total protein in the human body. This protein is widely used for cell cultureware coating due to its high biocompatibility. Actually, the collagen used for coating cell cultureware can fibrillate, such that collagen molecules self-assemble with a specific orientation, eventually forming a gel when the collagen is neutralised and incubated at 37 °C. Utilizing the property of gel formation by heating, 3D culture in a collagen gel can be performed with variously shaped cell cultureware platforms by combining a collagen solution with cells and medium and adding the mixture to the cell cultureware.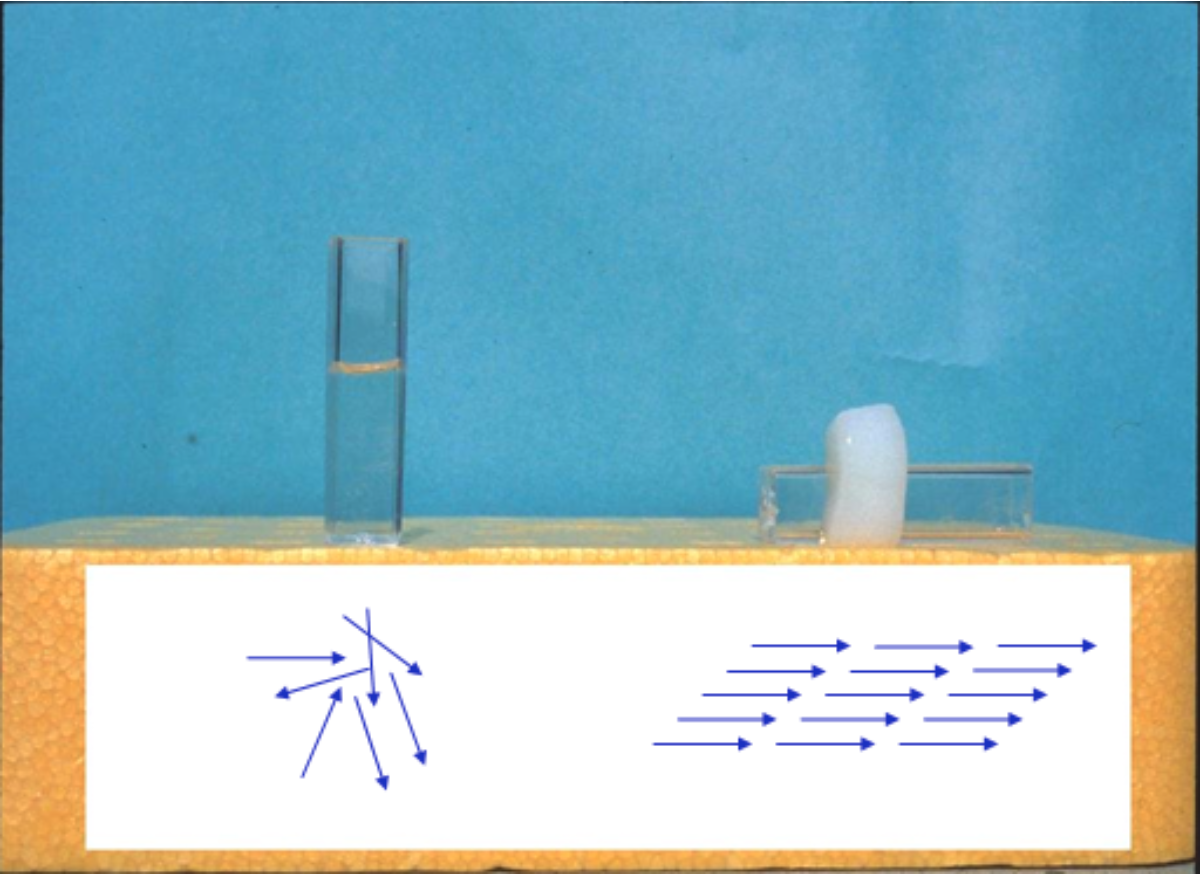
Collagen molecule alignment and gel formation by heating
Oxygen supply to cells subjected to 3D culturing in collagen gel
There also is a method for performing 3D culture by forming spheroids. However, as the spheroid diameter increases, cells in the centre of the spheroid tend to die, due in part to impaired exchange and decreased oxygen supply. On the other hand, collagen gel is considered to be capable of permitting 3D culture in an environment more closely resembling that in the living body. Experiments by Colom et al. showed that when pulmonary epithelial cells were cultured in 0.3% collagen gel for 46 hours, the oxygen partial pressure in the gel reached approximately 20 mmHg; for comparison, the oxygen partial pressure in most tissues such as liver and brain falls into within a range of 20 to 50 mmHg (1). Interestingly, in a similar experiment with 0.1% collagen gel, the oxygen partial pressure after 46 hours of culture was about 70 mmHg, a value close to the 75-100 mmHg oxygen partial pressure observed in arteries and alveoli.
Penetration of physiologically active substance added to collagen gels
While comparative evaluation of cell function by 3D culture and plate culture is the primary interest of many researchers, other scientists are interested in applying 3D culture for experiments using physiologically active substances. When added to the medium in plate culture, those substances are likely to diffuse relatively rapidly. In contrast, the diffusion of such substances is slowed in collagen gels, depending on collagen concentration and thickness. For instance, Galgoczy et al. calculated that the time until 4-kDa, 40-kDa, and 70-kDa substances reach half the equilibrium concentration in a 2-mm-thick 0.3% collagen gel containing cells is approximately 16 hours, 34 hours, and 46 hours, respectively (2). Those authors proposed considering whether the physiologically active substance possesses a binding domain for extracellular matrix components such as collagen, and/or adding physiologically active substances from above and below the collagen gel to accelerate the diffusion.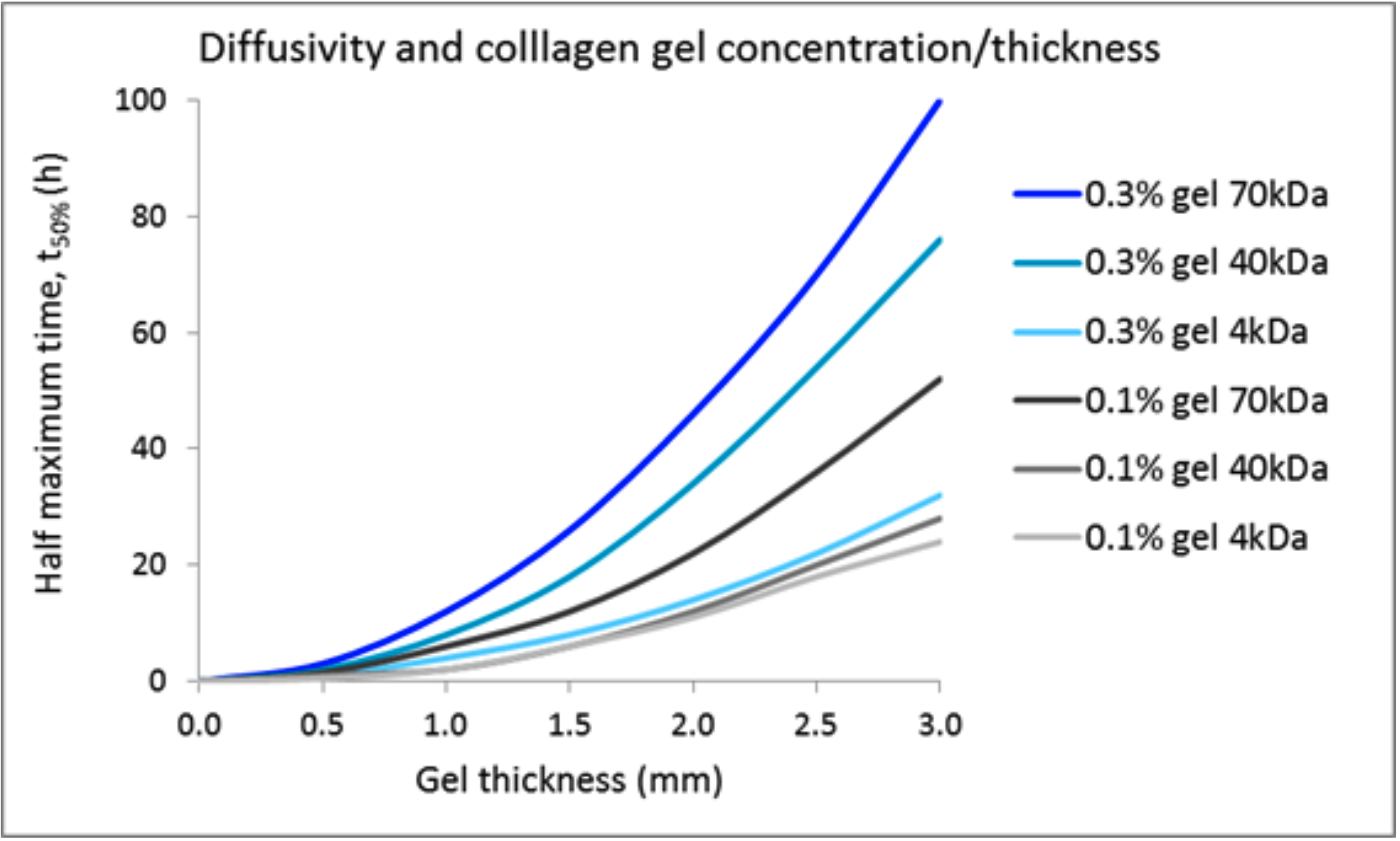
References:
1) Oxygen diffusion and consumption in extracellular matrix gels: Implications for designing three-dimensional cultures. Colom A, Galgoczy R, Almendros I, Xaubet A, Farré R, Alcaraz J.J Biomed Mater Res A. 2014 Aug;102(8):2776-84. PMID: 24027235
2) A spectrophotometer-based diffusivity assay reveals that diffusion hindrance of small molecules in extracellular matrix gels used in 3D cultures is dominated by viscous effects. Galgoczy R, Pastor I, Colom A, Giménez A, Mas F, Alcaraz J. Colloids Surf B Biointerfaces. 2014 Aug 1;120:200-7. PMID: 24916283
In vivo applications
 |
Atelocollagen Honeycomb Sponge |
|
 |
Sustained-release of bioactive compounds Atelocollagen Honeycomb Sponge |
|
 |
Collagen induced arthritis model Type II collagen Solution (3mg/ml) |
|
 |
 |
AteloGene(r) Whole Animal siRNA/miRNA Transfection Kits AteloGene Local Use, Quick Gelation (for 15 mice) |
FibColl® Atelocollagen Inserts 24
3D Honeycomb Boosted
3D Ready Atelocollagen
Atelocollagen / Native Collagen Acidic Solutions
Atelocollagen Neutral Solutions
Atelocollagen, Honeycomb Sponge
Atelocollagen Sponge, MIGHTY
Atelocollagen Coated B-TCP Scaffold
Atelocollagen Microspheres
Collagen Sponge for 35 mm Culture Dish
Atelocollagen, Permeable Membrane for 50 mm Culture Dish
Atelocollagen, Permeable Membrane for 6-well, 24-well Culture Plate
Type II Collagen
Atelocollagen Powder, Membrane, Sponge