this page: Exosome Dashboard
Product Lineup
Tetraspanin mAbs for exosome purification and characterization
ExoTrap™(to capture exosomes)
Ultracentrifugation media for exosome purification
OptiPrep™ (for exosome isolation)
Milk exosome reagents
Antibodies and ELISAs for putative exosome markers and negative controls
Other Resources
User Reports
Annotated Bibliography
EXOSOME Dashboard
Isolation and Characterization with
Anti-Human Tetraspanin mAbs and OptiPrep™
| What are exosomes? | |
| Extracellular vesicles (EVs; including classical-exosomes, non-classical-exosomes, microvesicles, large oncosomes, apoptotic bodies, apoptotic vesicles, autophagic extracellular vesicles, amphisomes and ARRMs) are membrane vesicles of 40-1000 nm that are released into the extracellular milieu and body fluids from most cell types, including red blood cells, platelets, lymphocytes, dendritic cells, endothelial cells and tumor cells. These vesicles are classified into 2 types according to their secretory mechanism. Thus, classical-exosomes are formed in multivesicular endosomes, whereas microvesicles originate by direct budding from the plasma membrane. Although classical-exosome components vary by their originating cell type, a certain set of molecules appears likely to be shared, regardless of their origin. These molecules include the tetraspanin proteins (CD9, CD63 and CD81) that are thought to be essential components of the biogenesis mechanism of classical-exosomes. Accordingly, researchers have used CD9, CD63 and CD81 to isolate and characterize the purity of classical-exosome preparations. |
Over the past decade, exosomes have been the focus of intense interest as microRNA (miRNA) carriers, disease biomarkers, and potential therapeutic drug delivery vehicles. Despite the importance of exosomes, their isolation and characterization are still considered major scientific challenges, especially when translating to the demands of the clinic. Classically, exosomes and other EVs have been purified by ultracentrifugation. Technical challenges, time and cost have led to a proliferation of alternative purification approaches, many of them now commercially available. While exosomal content has been reported to include genomic DNA, RNA, proteins, and lipids, recent studies have cast doubt as to whether DNA and many so-called “exosomal” proteins are actual exosomal constituents or simply non-vesicular contaminants co-isolated and comingled by ultracentrifugation forces at the bottom of centrifuge tubes.
A recent landmark paper (Jeppesen, D., Fenix, A., Franklin, J., Higginbotham, J., Zhang, Q., Zimmerman, L., Liebler, D., Ping, J., Liu, Q., Evans, R., Fissell, W., Patton, J., Rome, L., Burnette, D., Coffey, R. (2019). Reassessment of Exosome Composition Cell 177(2), 428-445.e18.) showed that the majority of exosome isolation approaches, including classical ultracentrifugation, suffer from significant protein and DNA contamination from non-vesicular extracellular sources. The methods used to reveal this surprising level of contamination include high resolution iodixanol density gradient ultracentrifugation [to separate small EVs from non-vesicular components (such as vaults and exomeres)] and direct immunocapture (DIC) from human plasma and cell conditioned media using antibodies to tetraspanin molecules CD63, CD9 or CD81.
High Quality anti-Human Tetraspanin Monoclonal Antibodies
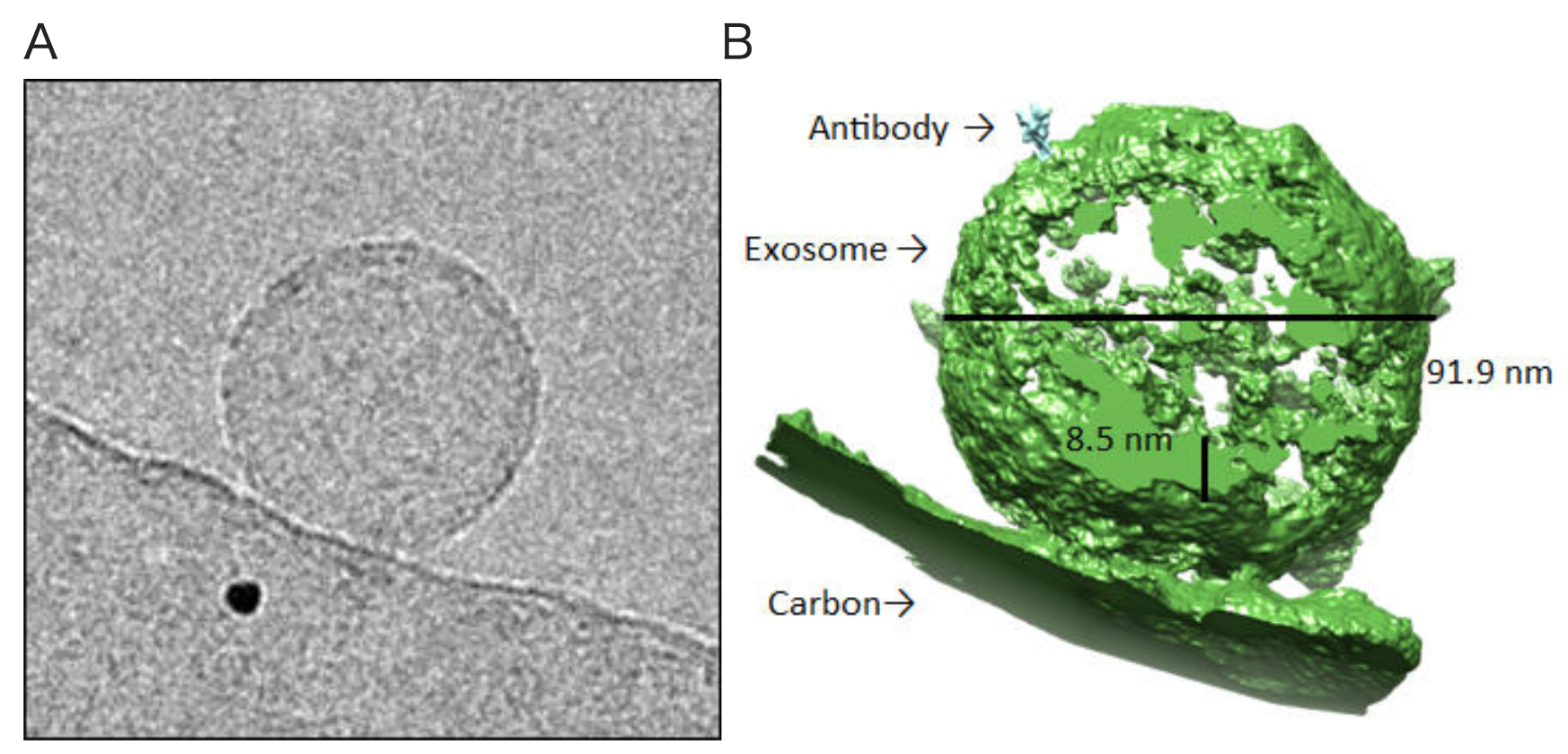 Cryo-electron microscopic detection of exosomes bound by anti-human CD63 antibody (clone 8A12).
Cryo-electron microscopic detection of exosomes bound by anti-human CD63 antibody (clone 8A12).Exosomes and anti-human CD63 antibody (clone 8A12) were mixed and imaged with cryo-electron microscopy (Titan Krios). Shown is a cryo-electron micrograph of an exosome (A), a three-dimensional reconstruction of an exosome (green) with a single bound antibody (cyan) (B) and a rotating 3D tomographic reconstruction of an exosome and a bound antibody (Top). Images courtesy of Okinawa Protein Tomography Ltd.
High specificity anti-human tetraspanin antibodies from CosmoBio USA are ideal reagents for characterizing and isolating classical exosomes using new “ultraclean” methods.
| Raised against tetraspanin extracellular loops | ||||
| Target | Clone | Immunogen | isotype | purification |
| CD9 | 12A12 | Amino acids 113-195: the large second extracellular loop between the 3rd and 4th transmembrane domains | IgG2b | Protein G |
| CD63 | 8A12 | Amino acids 104-202: the large second extracellular loop between the 3rd and 4th transmembrane domains | IgG2a | Protein G |
| CD81 | 12C4 | Amino acids 36-54: the small first extracellular loop between the 3rd and 4th transmembrane domains | IgG2a | Protein G |
| Human reactive | ||||
| Target | Clone | Reactive | Non-reactive | |
| CD9 | 12A12 | Human | Mouse, Rat | |
| CD63 | 8A12 | Human | Mouse, Rat | |
| CD81 | 12C4 | Human, Cow | Mouse, Rat | |
| High performance in multiple applications | |||
| Application | anti-CD9 (clone 12A12) | anti-CD63 (clone 8A12) | anti-CD81 (clone 12C4) |
| IP | + | + | + |
| WB | + | + | + |
| FC | + | + | + |
| ELISA | + | + | + |
| IF | + | + | + |
| IHC | + | + | + |
| IEM | + | + | + |
| ExoTrap™ Exosome Isolation Spin Column | + | - | - |
| CD9/CD63 Exosome ELISA Kit | + | + | - |
| ExoScreen assay | + | + | not tested |
| In vivo metastasis assay | + | not tested | not tested |
| Mass spectrometric immunoassay with monolith pipette tips | + | not tested | not tested |
| Membrane fusion assay | not tested | + | not tested |
| In vitro opsonization assay | + | + | not tested |
| Effective in multiple human biofluids | |||
| Biofluid | anti-CD9 (clone 12A12) |
anti-CD63 (clone 8A12) |
anti-CD81 (clone 12C4) |
| plasma | + | + | + |
| culture supernatant | + | + | + |
| urine | + | + | not tested |
| cerebrospinal fluid | + | not tested | not tested |
| pleural lavage | not tested | + | not tested |
| milk | not tested | not tested | + |
| liver tissue | not tested | not tested | + |
*Tide Fluor™ 2 (TF2) has spectral properties similar to those of fluorescein and Alexa Fluor® 488 (Invitrogen). Compared to fluorescein, TF2 has much stronger fluorescence and higher photostability. Additionally, its fluorescence is pH-independent from pH 3 to 11. TF2-labeled antibodies exhibit much stronger fluorescence and higher photostability than those labeled with FITC. These characteristics make TF2 a superior alternative to fluorescein.
#Tide Fluor™ 5 (TF5) has spectral properties essentially identical to those of Cy5. Compared to Cy5, TF5 has much stronger fluorescence and higher photostability. Additionally, its fluorescence is pH-independent from pH 3 to 11. TF5-labeled antibodies exhibit much stronger fluorescence and higher photostability than those labeled with Cy5. These characteristics make TF5 a superior alternative to Cy5.
| Conjugated to biotin, Tide Fluor-2* and Tide Fluor-4# | |
| anti-CD9 (clone 12A12) | unconjugated |
| biotin-conjugated | |
| Tide Fluor™ 2-conjugated | |
| Tide Fluor™ 4-conjugated | |
| anti-CD63 (clone 8A12) | unconjugated |
| biotin-conjugated | |
| Tide Fluor™ 2-conjugated | |
| Tide Fluor™ 4-conjugated | |
| anti-CD81 (clone 4C12) | unconjugated |
| biotin-conjugated | |
| Tide Fluor™ 2-conjugated | |
| Tide Fluor™ 4-conjugated | |
Ultraclean direct immunoaffinity capture (DIC) of classical-exosomes with tetraspanin antibodies
Immediately after collection, cell-conditioned medium was subjected to differential centrifugation at 400 x g, 2,000 x g and 15,000 x g at 4°C. The supernatant was filtered through a 0.22mm pore PES filter (Millipore) to generate pre-cleared medium. All following steps were also performed at 4°C. DIC of exosomes directly from human plasma was performed as described above except that the supernatant in these cases was raw, undiluted and unfiltered, plasma (generated by two rounds of 3000 x g centrifugation for 15 min). Pre-cleared medium was split three ways: one portion was incubated with magnetic beads directly conjugated to antibodies directed at CD63, CD81 or CD9; a second portion was incubated with magnetic beads conjugated to mouse IgG, and incubation was allowed to proceed with nutation and rotation for 16h; the third portion was subjected to ultracentrifugation and washing to generate a crude sEV pellet (P120). After incubation, the beads were washed four times in ice-cold 0.1% BSA-PBS (pH 7.4, filtered through a 0.22mm membrane), and finally, washed one time in PBS pH 7.4 (pH 7.4, filtered through a 0.22mm membrane). Immediately following the last wash, the exosome-loaded beads were resuspended first in cell lysis buffer and LDS sample buffer for 30 min on ice, and then resuspended in LDS sample buffer followed by heating to 70°C for 10 min to release proteins. The beads were removed from the suspension by using a magnet and the clarified lysate used for immunoblot analysis. In some cases, immediately following the last wash step, the exosome-loaded beads were lysed for DNA extraction.
(from: Jeppesen, D., Fenix, A., Franklin, J., Higginbotham, J., Zhang, Q., Zimmerman, L., Liebler, D., Ping, J., Liu, Q., Evans, R., Fissell, W., Patton, J., Rome, L., Burnette, D., Coffey, R. (2019). Reassessment of Exosome Composition Cell 177(2), 428-445.e18.)Click for Tetraspanin mAb User Reports
Ordering Information
| Product | Clone | Label | Unit size | Catalog Number |
|---|---|---|---|---|
| Tetraspanin antibodies for exosome purification and characterization | ||||
| Anti CD9 Antigen (MRP-1/Tspan-29) mAb | 12A12 | - | 50 UL | CAC-SHI-EXO-M01-50 |
| Anti CD9 Antigen (MRP-1/Tspan-29) mAb | 12A12 | - | 100 UL | CAC-SHI-EXO-M01-100 |
| Anti CD9 Antigen (MRP-1/Tspan-29) mAb | 12A12 | biotin | 100 UL | CAC-SHI-EXO-M01-B |
| Anti CD9 Antigen (MRP-1/Tspan-29) mAb | 12A12 | TF | 100 UL | CAC-SHI-EXO-M01-TF2 |
| Anti CD9 Antigen (MRP-1/Tspan-29) mAb | 12A12 | TF5 | 100 UL | CAC-SHI-EXO-M01-TF5 |
| Anti CD63 Antigen (LAMP-3/Tspan-30) mAb | 8A12 | - | 50 UL | CAC-SHI-EXO-M02-50 |
| Anti CD63 Antigen (LAMP-3/Tspan-30) mAb | 8A12 | - | 100 UL | CAC-SHI-EXO-M02-100 |
| Anti CD63 Antigen (LAMP-3/Tspan-30) mAb | 8A12 | biotin | 100 UL | CAC-SHI-EXO-M02-B |
| Anti CD63 Antigen (LAMP-3/Tspan-30) mAb | 8A12 | TF2 | 100 UL | CAC-SHI-EXO-M02-TF2 |
| Anti CD63 Antigen (LAMP-3/Tspan-30) mAb | 8A12 | TF5 | 100 UL | CAC-SHI-EXO-M02-TF5 |
| Anti CD81 Antigen (TAPA-1/Tspan-28) mAb | 12C4 | - | 50 UL | CAC-SHI-EXO-M03-50 |
| Anti CD81 Antigen (TAPA-1/Tspan-28) mAb | 12C4 | - | 100 UL | CAC-SHI-EXO-M03-100 |
| Anti CD81 Antigen (TAPA-1/Tspan-28) mAb | 12C4 | biotin | 100 UL | CAC-SHI-EXO-M03-B |
| Anti CD81 Antigen (TAPA-1/Tspan-28) mAb | 12C4 | TF2 | 100 UL | CAC-SHI-EXO-M03-TF2 |
| Anti CD81 Antigen (TAPA-1/Tspan-28) mAb | 12C4 | TF5 | 100 UL | CAC-SHI-EXO-M03-TF5 |
| Tetraspanin antibody-based exosome purification and ELISA kit | ||||
| ExoTrap™ Exosome Isolation Spin Column Kit | 12A12 | - | 10 preps | CSR-SHI-EXO-K010 |
| CD9/CD63 Exosome ELISA Kit | 12A12/8A12 | - | 1 Kit | CSR-EXH0102EL |
| Ultracentrifugation media for exosome purification | ||||
| OptiPrep™-Density Gradient Media (Iodixanol) | - | - | 1 x 250 ML | AXS-1114542 |
| OptiPrep™-Density Gradient Media (Iodixanol) | - | - | 5 x 250 ML | AXS-1114542-5 |
| Milk exosome reagents | ||||
| Anti-Bovine Milk Exosome pAb | - | - | 100 UL | CAC-EXO-AB-01 |
| Human milk-derived exosomes | - | - | 1 ML | CSR-EXHM100L |
| Antibodies and ELISAs for putative exosome markers and negative controls | ||||
| Human TSG101 ELISA Kit | - | - | 1 x 96 Rxns | CSB-EL025125HU |
| Human ALIX ELISA Kit | - | - | 1 x 96 Rxns | CSB-EL017673HU |
| Human Flotillin-1 (FLOT1) ELISA Kit | - | - | 1 x 96 Rxns | CSB-EL008727HU |
| Human HSP70 ELISA Kit | - | - | 1 x 96 Rxns | CSB-E08297h |
| Human GAPDH ELISA Kit | - | - | 1 x 96 Rxns | CSB-E13911h |
| Anti Human EPCAM mAb | hrk29 | - | 50 UG | CAC-HT-MAB1 |
| Human EPCAM ELISA kit | - | - | 1 x 96 Rxns | CSB-EL007717HU |
| Anti Vimentin pAb (Rabbit, Antiserum) | - | - | 100 UL | LSL-LB-3010 |
| Human Vimentin ELISA Kit | - | - | 1 x 96 Rxns | CSB-E08982h |
| Human CETP ELISA Kit | - | - | 1 x 96 Rxns | CSB-E08567h |
| Human CXCR4 ELISA Kit | - | - | 1 x 96 Rxns | CSB-E12825h |
| Anti CD68 mAb | PM-1K | - | 50 UG | KAL-KT117 |
| Human CD68 ELISA Kit | - | - | 1 x 96 Rxns | CSB-E15979h |
| Anti CD44 Antigen v9 mAb | RV3 | - | 50 UG | CAC-LKG-M003 |
| Anti CD44 Antigen v9 mAb | RV3 | - | 100 UG | CAC-LKG-M001 |
| Human CD44 ELISA Kit | - | - | 1 x 96 Rxns | CSB-E11846h |
| Human HLA-G ELISA Kit | - | - | 1 x 96 Rxns | CSB-E09401h |
| Human HSPA8 ELISA Kit | - | - | 1 x 96 Rxns | CSB-EL010829HU |
