Click here to review scores of density gradient media-related general references, protocols, literature references. Download application sheets for viruses, cells, subcellular fractionation, and marcromolecules and macromolecular complexes.
Click here to access to the Density Gradient Media Dashboard Page
Click here for more information and to see all exosome related products from Cosmo Bio USA.
OptiPrep™ (iodixanol solution)
Nycodenz® iohexol powder)
Lymphoprep™
Lymphoprep Tube™
Polymorphprep™
Polysucrose™ 400
Lymphoprep™-related Documents
Lymphoprep Package Insert
Application Overviews
Analysis of Membrane Trafficking
Isolation of Blood Cells
Isolation of Cells
Isolation of Exosomes
Isolation of Macromolecules and Lipoproteins
Isolation of Plasma Membranes
Isolation of Cell Organelles
Isolation of Viruses
Fantastic Resource
Browse +100 Application Data Sheets and Citation Lists for Cells, Subcellular Components, Macromolecules, and Viruses
See all Density Gradient Media Products

Lymphoprep™ is a ready-made, sterile, endotoxin-tested, separation medium for isolating human mononuclear cells (lymphocytes and monocytes), especially from blood. Relatively low buoyant density (below 1.077 g/ml) mononuclear cells are retained at the sample/medium interface while more dense erythrocytes and polymorphonuclear leukocytes (PMNs or granulocytes) sediment through the medium.
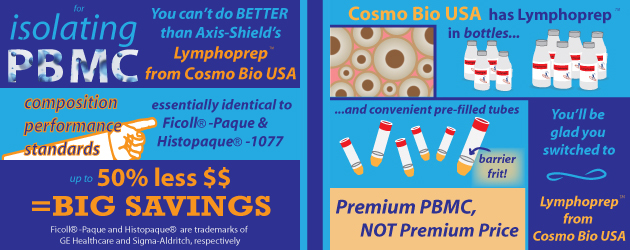
Why Payque more?
Lymphoprep™ has the same specifications as Ficoll(R)-Paque and Histopaque(R)-1077 and is manufactured to essentially equivalent quality standards. As compared to top brands, Lymphoprep™ can save up to 50% on gradient medium.
About Lymphoprep™
A simple and effective method for the isolation of mononuclear cells from human blood was reported by Dr. Arne Bøyum in 1968. For more than 35 years Lymphoprep™ medium has been widely used for isolating mononuclear cells (monocytes and lymphocytes) by exploiting their low buoyant density (below 1.077 g/ml) as compared to erythrocytes and polymorphonuclear (PMN) leukocytes (granulocytes). Mononuclear cells are retained at the sample/medium interface while erythrocytes and PMNs sediment through the medium.
The described method is rapid, simple and reliable and gives excellent results with blood samples from normal individuals and patients. To obtain maximum yields it is important that blood samples are diluted 1:1 with physiological saline before being applied to Lymphoprep™. Erythrocyte contamination in mononuclear cell suspensions is usually between 3-10% of the total cell number. Some immature PMNs may sediment with lymphocytes during intense immunosuppressive therapy. Removal of most platelets from mononuclear cell preparations is essential to avoid inhibition in cytotoxicity tests. Lymphoprep™ can be used for the preparation of pure lymphocyte suspensions for tissue typing, anti-lymphocyte sera and immunological research. Thorsby and Brattelie used this technique with only slight modifications in the preparation of pure lymphocyte suspensions for cytotoxicity tests and lymphocyte cultures.
Each batch of Lymphoprep™ is assessed for endotoxin level using a specific LAL test. Axis-Shield's goal is to produce batches with an endotoxin level lower or equal to 0.13 EU/ml.
Specifications
- Sodium diatrizoate 9.1% (w/v)
- Polysaccharide 5.7% (w/v)
- Density 1.077 ± 0.001 g/mL
- Osmolality 290 ± 15 mOsm
- Endotoxin <1.0 EU/mL
- Sterility claimed according to Ph.Eur
Lymphoprep™ is manufactured, packaged and released in compliance with:
- Current EU Guide to Good Manufacturing Practice
- Fresenius Kabi AS Quality System
- Fresenius Kabi AS Manufacturing License
For every lot, a Certificate of Analysis showing the actual values for density, osmolality and endotoxin level is made available at www.axis-shield-density-gradient -media.com.
Essential manufacturer's information
| Documents & Links for Lymphoprep | |
| MSDS | Lymphoprep MSDS |
| Datasheet | Lymphoprep Package Insert |
| Application Note | Purification of mononuclear cells, monocytes and polymorphonuclear leukocytes |
| Application Note | Lymphoprep, Isolation of human mononuclear cells |
| Documents & Links for Lymphoprep | |
| MSDS | Lymphoprep MSDS |
| Datasheet | Lymphoprep Package Insert |
| Application Note | Purification of mononuclear cells, monocytes and polymorphonuclear leukocytes |
| Application Note | Lymphoprep, Isolation of human mononuclear cells |
| Citations for Lymphoprep – 1 Found |
| Maeta et al. 2019. Lymphokine-activated killer cell transplantation after anti-cancer treatment in two aged cats. Open Vet J. 9(2):147-150. Journal |











